Part:BBa_J61100
Ribosome Binding Site Family Member
.
Parts J61100-J61150 are a family of similar ribosome binding site basic parts identified from a saturation mutagenic library.
Library TCTAGAGAAAGANNNGANNNACTAGT J61100 tctagaGAAAGAGGGGACAAactagt J61101 tctagaGAAAGACAGGACCCactagt J61102 tctagaGAAAGATCCGATGTactagt J61103 tctagaGAAAGATTAGACAAactagt J61104 tctagaGAAAGAAGGGACAGactagt J61105 tctagaGAAAGACATGACGTactagt J61106 tctagaGAAAGATAGGAGACactagt J61107 tctagaGAAAGAAGAGACTCactagt J61108 tctagaGAAAGACGAGATATactagt J61109 tctagaGAAAGACTGGAGACactagt J61110 tctagaGAAAGAGGCGAATTactagt J61111 tctagaGAAAGAGGCGATACactagt J61112 tctagaGAAAGAGGTGACATactagt J61113 tctagaGAAAGAGTGGAAAAactagt J61114 tctagaGAAAGATGAGAAGAactagt J61115 tctagaGAAAGAAGGGATACactagt J61116 tctagaGAAAGACATGAGGCactagt J61117 tctagaGAAAGACATGAGTTactagt J61118 tctagaGAAAGAGACGAATCactagt J61119 tctagaGAAAGATTTGATATactagt J61120 tctagaGAAAGACGCGAGAAactagt J61121 tctagaGAAAGAGACGAGTCactagt J61122 tctagaGAAAGAGAGGAGCCactagt J61123 tctagaGAAAGAGATGACTAactagt J61124 tctagaGAAAGAGCCGACATactagt J61125 tctagaGAAAGAGCCGAGTTactagt J61126 tctagaGAAAGAGGTGACTCactagt J61127 tctagaGAAAGAGTGGAACTactagt J61128 tctagaGAAAGATAGGACTCactagt J61129 tctagaGAAAGATTGGACGTactagt J61130 tctagaGAAAGAAACGACATactagt J61131 tctagaGAAAGAACCGAATTactagt J61132 tctagaGAAAGACAGGATTAactagt J61133 tctagaGAAAGACCCGAGACactagt J61134 tctagaGAAAGACCGGAAATactagt J61135 tctagaGAAAGACCGGAGACactagt J61136 tctagaGAAAGAGCTGAGCAactagt J61137 tctagaGAAAGAGTAGATCAactagt J61138 tctagaGAAAGATATGAATAactagt J61139 tctagaGAAAGATTAGAGTCactagt
These parts are present in plasmid pSB1A2, but there is also a constitutive promoter (J23100-derived) inserted into the XbaI site. So, for example, the EcoRI/PstI region of part J61100 reads:
Biobrick 5' XbaI J23100 XbaI RBS Part Biobrick 3' gaattcgcggccgcttctagaGTTGACGGCTAGCTCAGTCCTAGGTACAGTGCTAGCTtctagaGAAAGAGGGGACAAactagtagcggccgctgcag
This feature in no way prevents the use of these parts in standard Biobrick assembly. Normal prefix insertion into EcoRI/XbaI will delete this promoter element. Suffix insertion into SpeI/PstI will retain this promoter, but it can of course be removed later by a prefix insertion.
Note also that the base 5' to the SpeI site is allowed to float in these parts and is therefore rarely "T". The "G" downstream of the XbaI site obeys the standard. Because the database does not permit variation at this position, the predicted sequences of composite parts derived from these parts will be incorrect at this position.
More on this family of parts and their quantitative behavior is described here.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Team Warsaw 2010's measurement
RBS strength (relative to B0034): 4,29%Team TU Delft 2010's measurements
RBS strength: 2.0%, standard deviation: 0.512%RBS strength is relative to B0034, obtained from an average of 12 measurements. Protein production rate is calculated using our production model
Contribution
Group: Valencia_UPV iGEM 2018
Author: Adrián Requena Gutiérrez, Carolina Ropero
Summary: We adapted the part to be able to assemble transcriptional units with the Golden Gate assembly method
Documentation:
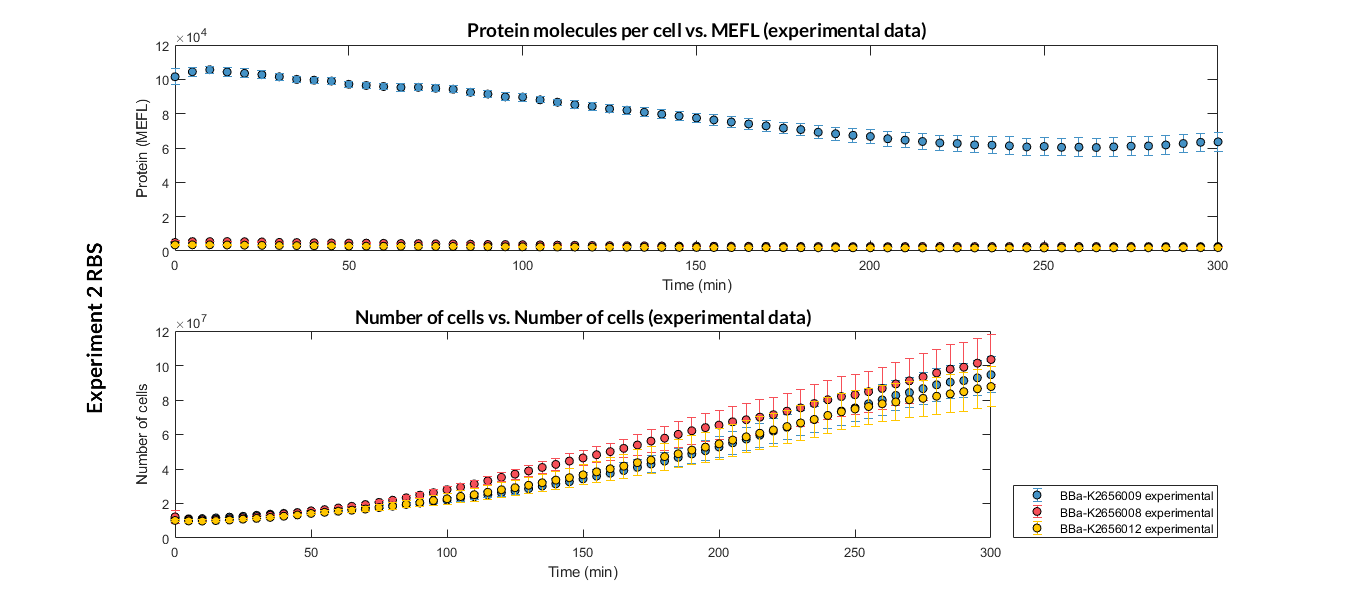
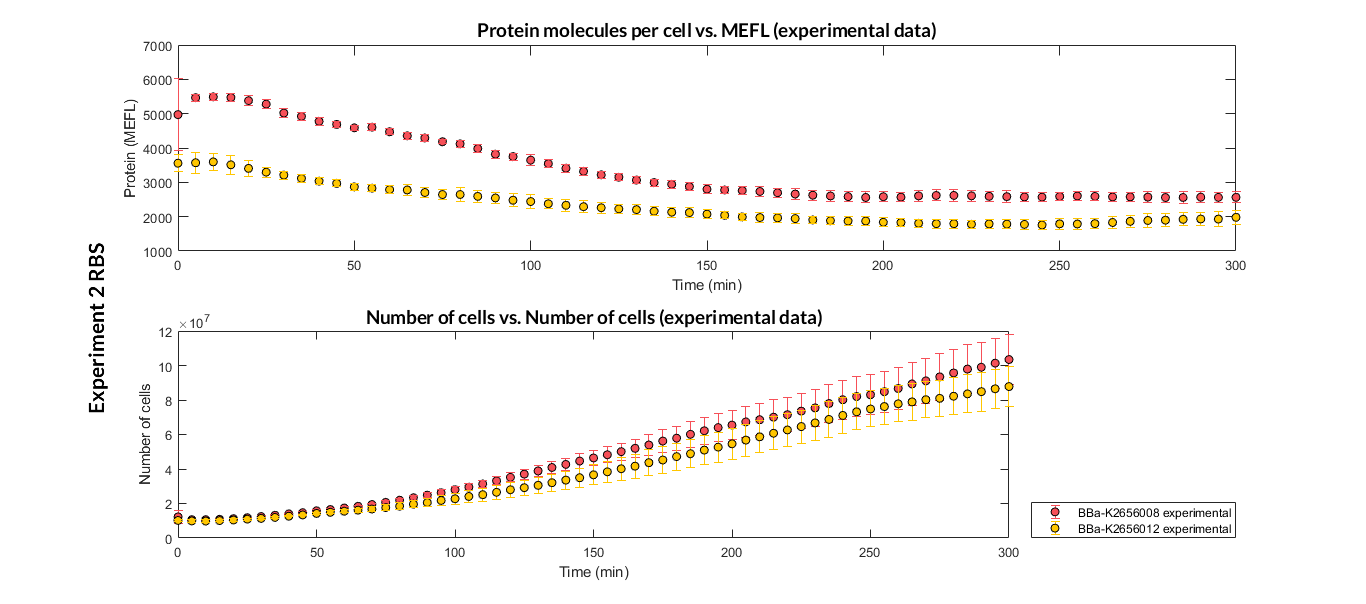
In order to create our complete [http://2018.igem.org/Team:Valencia_UPV/Part_Collection part collection] of parts compatible with the Golden Gate assembly method, we made the part BBa_K2656008 which is this part adapted to the Golden Gate technology.
Characterization of the this part was performed with the transcriptional unit BBa_K2656100, which was used in a comparative RBS expression experiment with composite parts BBa_K2656104 and BBa_K2656101. They all were assembled in a Golden Braid alpha1 plasmid using the same promoter, CDS and terminator.
By using this [http://2018.igem.org/Team:Valencia_UPV/Experiments#exp_protocol experimental protocol], we have obtained the parameters to valide our [http://2018.igem.org/Team:Valencia_UPV/Modeling#models constitutive model]and rationale choose its optimized values based for each RBS.
| Table 1. Optimized parameters for the BBa_K2656008 RBS. | |||
| Parameter | Value | ||
| Translation rate p | p = 0.04089 min-1 | ||
| Dilution rate μ | μ = 0.01288 min-1 | ||
We have also calculated the relative force between the different RBS, taking BBa_K2656009 strong RBS as a reference. Likewise, a ratio between p parameters of the different RBS parts and p parameter of the reference RBS has been calculated.
| Table 2. BBa_K2656008 relative strength and p ratio. | |||
| Parameter | Value | ||
| Relative strength | 0.042 | ||
| p parameter ratio (pRBS/pref) | 0.044 | ||
>Internal Priming Screening Characterization of BBa_J61100: Has no possible internal priming sites between this BioBrick part and the VF2 or the VR primer.
The 2018 Hawaii iGEM team evaluated the 40 most frequently used BioBricks and ran them through an internal priming screening process that we developed using the BLAST program tool. Out of the 40 BioBricks we evaluated, 10 of them showed possible internal priming of either the VF2 or VR primers and sometime even both. The data set has a range of sequence lengths from as small as 12 bases to as large as 1,210 bases. We experienced the issue of possible internal priming during the sequence verification process of our own BBa_K2574001 BioBrick and in the cloning process to express the part as a fusion protein. BBa_K2574001 is a composite part containing a VLP forming Gag protein sequence attached to a frequently used RFP part (BBa_E1010). We conducted a PCR amplification of the Gag-RFP insert using the VF2 and VR primers on the ligation product (pSB1C3 ligated to the Gag + RFP). This amplicon would serve as template for another PCR where we would add the NcoI and BamHI restriction enzyme sites through new primers for ligation into pET14b and subsequent induced expression. Despite gel confirming a rather large, approximately 2.1 kb insert band, our sequencing results with the VR primer and BamHI RFP reverse primer gave mixed results. Both should have displayed the end of the RFP, but the VR primer revealed the end of the Gag. Analysis of the VR primer on the Gag-RFP sequence revealed several sites where the VR primer could have annealed with ~9 - 12 bp of complementarity. Internal priming of forward and reverse primers can be detrimental to an iGEM project because you can never be sure if the desired construct was correctly inserted into the BioBrick plasmid without a successful sequence verification.
Team UChicago 2019's measurement
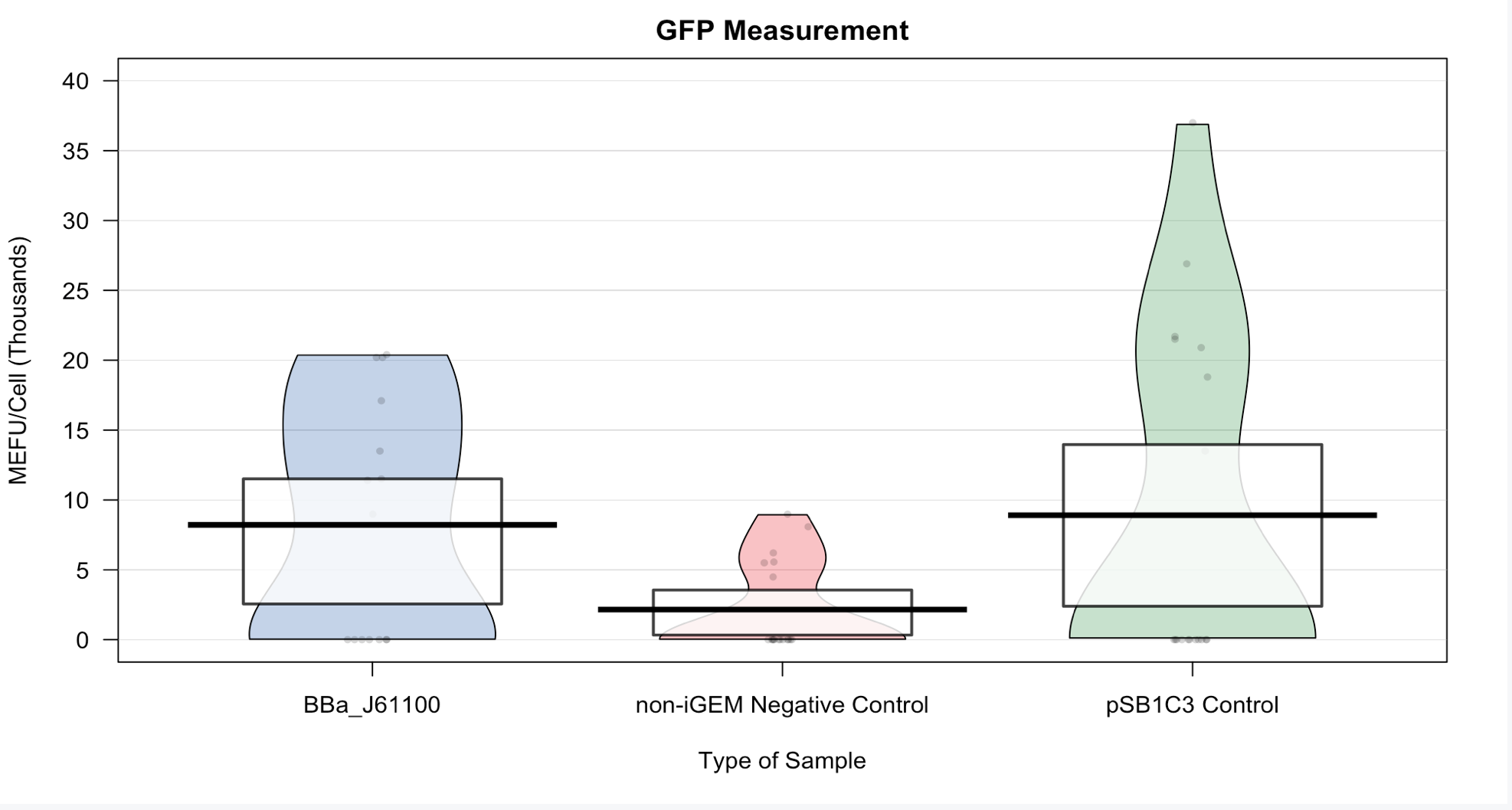
The Strength of this ribosome binding site has previously been shown to be extremely week, but up to this point, it has not been evaluated using the protocol that was designed as a result of the 2018 Interlab Study. UChicago set out to characterize this RBS using the Interlab Protocol. To do this, we attached this promoter RBS construct to BBa_I746916 which is a super folder GFP. We then followed the Protocol for a plate reader experiment using this construct, a blank pSB1C3 vector, and a blank non-iGEM vector.
From the plot below, we can definitely see that there are small amounts of green fluorescent protein (gfp) present in our ligation sample. We have chosen to use fluorescent measurement as a means to measure our ribosome binding site efficiency because the ribosome binding sites connect the promoter with an iGEM gfp. Since there is gfp present, this means that our characterization was successful. Our empty vector control shows small amounts of gfp due to the nature of our promoter BBa_J23119. Our negative control shows similar readings as the background readings on our plate reader, which means that our negative control is successful because there are no gfp present. This serves as a good reference for our sample comparison. The finding that there is no significant difference between our negative control and the gfp part in fluorescence confirms team Delft's measurements that this ribosome binding site is weak(found here). Furthermore, a non-iGEM vector (adapted from Rauch et al, 2019) showed lower fluorescence than did the iGEM vector with and without the gfp gene inserted. This indicates some auto-fluorescence of the iGEM vector which limits the feasibility of the 2018 interlab protocol when characterizing the fluorescence of low efficiency parts.
Functional Parameters: Austin_UTexas
Burden Imposed by this Part:

Burden is the percent reduction in the growth rate of E. coli cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the BBa_K3174002 - BBa_K3174007 pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the 2019 Austin_UTexas team.
This functional parameter was added by the 2020 Austin_UTexas team.
//ribosome/prokaryote/ecoli
//chassis/prokaryote/ecoli
//direction/forward
//regulation/constitutive
| None |

 1 Registry Star
1 Registry Star