Part:BBa_E0430
EYFP (RBS+ LVA- TERM) (B0034.E0030.B0015)
Standard YFP Output Device -LVA tag
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Functional Parameters
| biology | -NA- |
| emission | Yellow |
| tag | None |
| type | Reporter |
Team USP-Brazil 2018 Improvement
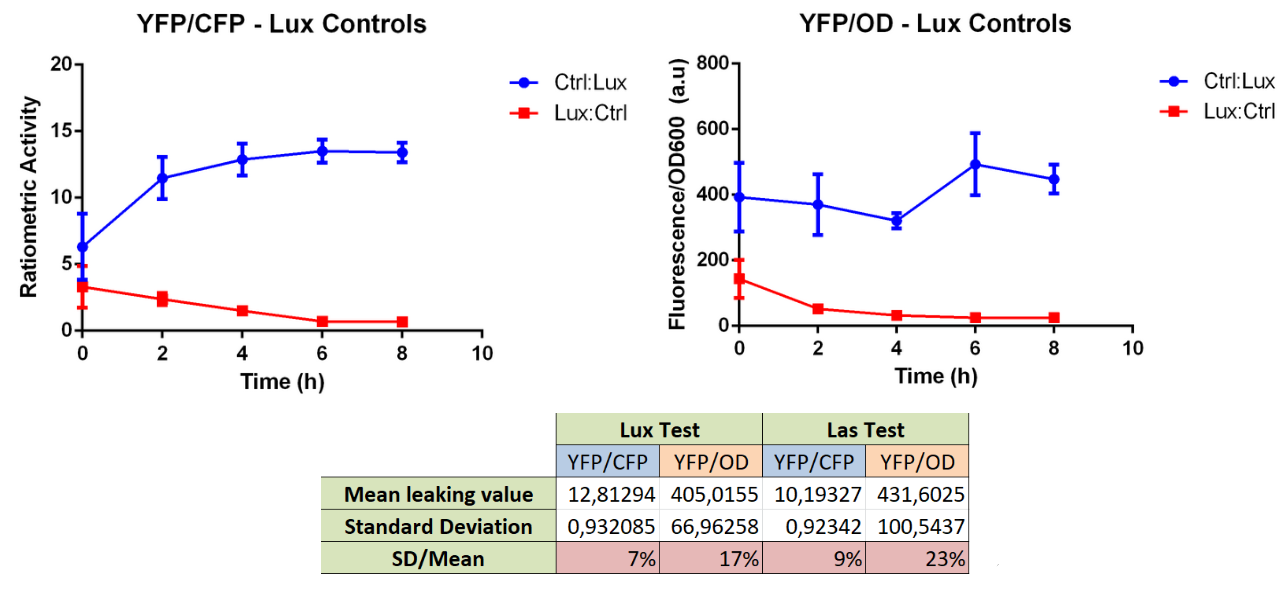
Team USP-Brazil, in 2018, has improved this part (BBa_K2771020) by adding it to a control gene, being that CFP constitutively expressed by ptet, which serves as a proxy for external influences on the first reporter's measurement, such as cell density, plasmid copy number and ,to some amount, metabolic burden:
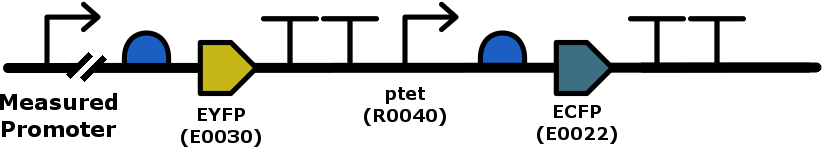
This improvement has shown to give better measurements when normalizing YFP output by dividing by CFP fluorescence than when just measuring YFP and dividing that value by the measured OD600. This gives us the possibility of a much more reproducible result, less dependant on growth media and cell density (a rather unreliable parameter to measure) at the time of each measurement. Another good point for using this is that dividing fluorescence measurements gives us an adimensional parameter, that has greater capacity for comparation with other experiments and constructs. In our measurements of quorum-sensing-responsive promoters activity, we compared the variance of our controls, which should have a constant value for fluorescence due to a constant amount of leakiness, when normalizing by the OD600 value and the CFP fluorescence measurement. We found, in this and other experiments, that the normalization using CFP presented significatively less variance. In this example, we found a variance that represented, when reaching equilibrium value, a percentage of the mean twice as small as with the normalization with the OD.
Team: Tianjin 2019 characterization
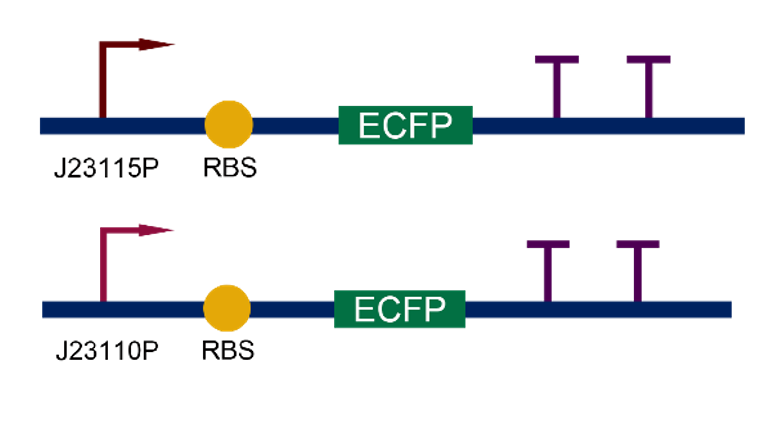
Team:Tianjin 2019 contributed to the reporter's characterization by connecting it to the combined promoters J23110 and J23115, assembling and testing their fluorescence intensity and detailed fluorescence excitation curves.
We linked the constructed fragment to the plasmid pRS415 and transformed it into Match 1 strain. Under normal culture conditions (LB+amp solid medium, 37℃), the Match 1 strain was cultured overnight. After about 8 hours, the colony was visible to the naked eye, with no obvious difference in appearance from ordinary e. coli (EYFP was not visible to the naked eye).
The experiment design
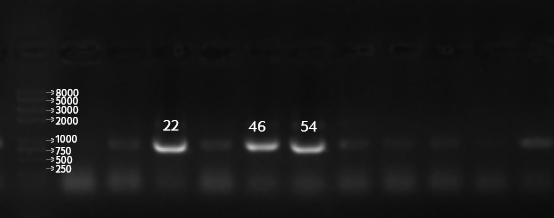
1.PCR validation
Because EYFP was not visible with the naked eye, we performed colony PCR validation to confirm whether the transformation was successful.
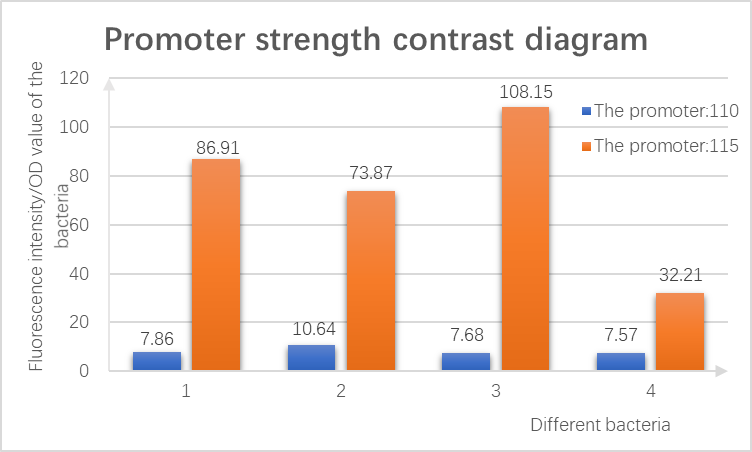
2.Determination by fluorescence microplate reader
The bacteria verified in the previous step were cultured separately overnight, then a small number of bacteria were selected and suspended in 200μL ddH2O, and the fluorescence intensity of the same bacteria was measured under emission/excitation of 527/514nm (reference value).
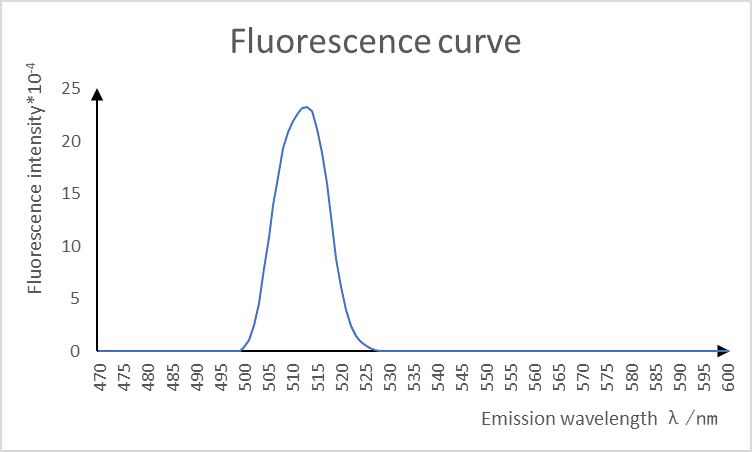
3. Fluorescence curve measurement
Under the condition of fixed excitation wavelength (512nm), we continuously measured its specific fluorescence intensity at the emission wavelength of 470~600nm, and obtained the following results.
Functional Parameters: Austin_UTexas
Burden Imposed by this Part:

Burden is the percent reduction in the growth rate of E. coli cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This part exhibited a significant burden. Users should be aware that BioBricks with a burden of >20-30% may be susceptible to mutating to become less functional or nonfunctional as an evolutionary consequence of this fitness cost. This risk increases as they used for more bacterial cell divisions or in larger cultures. Users should be especially careful when combining multiple burdensome parts, as plasmids with a total burden of >40% are expected to mutate so quickly that they become unclonable. Refer to any one of the BBa_K3174002 - BBa_K3174007 pages for more information on the methods and other conclusions from a large-scale measurement project conducted by the 2019 Austin_UTexas team.
This functional parameter was added by the 2020 Austin_UTexas team.
| biology | |
| emission | Yellow |
| tag | None |
| type | Reporter |

 1 Registry Star
1 Registry Star