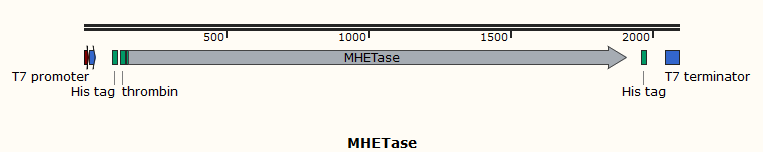
Part:BBa_K3997005
pET28a-MHETase-His
Profile
Name: pET28a-MHETase-His
Base Pairs: 1979 bp
Origin: Ideonella sakaiensis 201-F6, synthetic
Properties: Polyethylene terephthalate degradation enzyme
Usage and Biology
Polyethylene terephthalate (PET) is the most widely produced polyester plastic and its accumulation in the environment has become a global concern. At the same time, the daily intake of microplastics by humans is gradually increasing, which damages human health. Therefore, researchers believe that it is important to develop an environmental-friendly plastic degradation method by using microorganisms. Recently, a novel bacterial strain called Ideonella sakaiensis 201-F6 has been discovered that produces a couple of unique enzymes, PETase and MHETase, enabling the bacteria to utilize PET as their sole carbon source.
Figure 1. Action and function of MHETase in MHET degradation.
The enzyme MHETase is a hydrolase, and it represents a key step in the process of microbial PET degradation in I. sakaiensis. It cleaves monoterephthalic acid, the PET degradation product by PETase, to ethylene glycol and terephthalic acid, and it is crucial for hydrolysis of
MHET. We attempt to express the MHETase in E. coli strain to express and purify the protein to test its activity of degradation the MHET (Figure 1). So as to set up a method of environmental-friendly plastic degradation.
Construct design
The enzyme MHETase is a hydrolase, and it is crucial for hydrolysis of MHET. To verify this property, we use E. coli as the starting strain and construct an engineered strain of MHETase to explore its biological activity of the hydrolysis of MHET. To purify the protein, we also transfer the plasmid expressing MHETase into BL21(DE3) with a 6×His tag at it’s N-terminal. The enzyme is under the regulation of T7 promoter and can be induced by adding IPTG.
The T7 promoter is often used for protein overexpression. It is powerful and specific. It is completely controlled by T7 RNAP. When T7 RNAP is present in the cell, the T7 expression system occupies an absolute advantage compared to the host expression system. Its expression The speed is 5 times that of the former.
BBa_K3997001
Name: 6×His
Base Pairs: 18bp
Origin: synthetic
Properties: Polyhistidine tag
Usage and Biology
It is an polyhistidine tag, which is used in the purification of recombinant proteins
BBa_K3997001
Name: MHETase
Base Pairs: 1752 bp
Origin: Ideonella sakaiensis 201-F6
Properties: hydrolysis of MHET.
Experimental approach
Production, purification, and activity analysis of MHETase
1. Construction of pET28a-MHETase
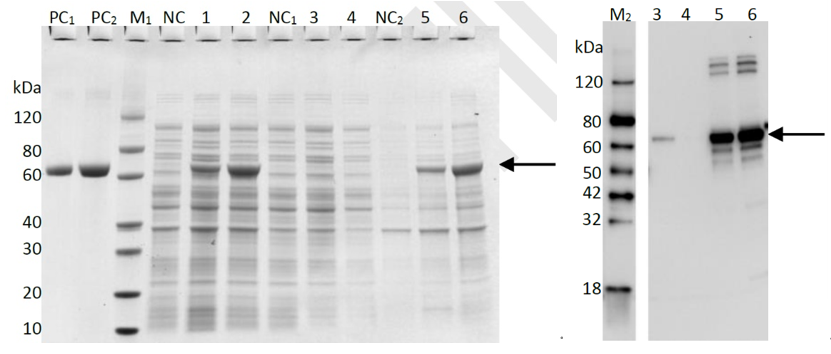
2. Purification of MHETase( ~65.17 kDa)_BL21(DE3) In order to present the function of the part, the MHETase gene was expressed in E. coli under the control of T7 promoter. Then the bacterial cells are collected and crushed, The samples of whole expression cell lysate, supernatant and pellet of cell lysate were analyzed using SDS-PAGE and Western blot (only for his-tag proteins from pET28a vector), which is found in the corresponding protein band of approximately 65kDa (Figure 3).
Lane M1: Protein marker
Lane M2: Western blot marker
Lane PC1: BSA (1μg)
Lane PC2: BSA (2μg)
Lane NC: Cell lysate without induction
Lane 1: Cell lysate with induction for 16h at 15oC
Lane 2: Cell lysate with induction for 4 h at 37oC
Lane NC1: Supernatant of cell lysate without induction
Lane 3: Supernatant of cell lysate with induction for 16h at 15oC
Lane 4: Supernatant of cell lysate with induction for 4 h at 37oC
Lane NC2: Pellet of cell lysate without induction
Lane 5: Pellet of cell lysate with induction for 16h at 15oC
Lane 6: Pellet of cell lysate with induction for 4 h at 37oC
The primary antibody for western blot is anti-His antibody
In addition, MHETase genes were also cloned to the expression vector pGEX-6P-1, which produce recombinant protein fusion with Glutathione-S-transferase (GST) tag.
Lane 1: MHETase Cell lysate without induction for 20 h at 16oC
Lane 2: MHETase Cell lysate with induction for 20 h at 16oC
Lane 3,4,5: GSH elution fractions of purification of lane 2 by GST-affinity chromatography
Proof of function
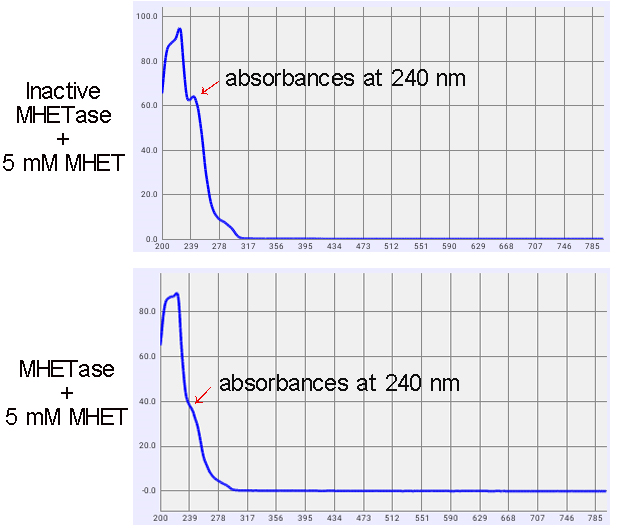
1. Enzyme Activity Tests
The activity of MHETase was indicated by the decline absorbances at a wavelength of 240 nm (Figure 4). The wavelength is the specific absorption of MHET. As shown in figure 2, after 1 d reaction, MHET concentration was dropped by 31.4%.
Improvement of an existing part
Compared to the old part BBa_K2013003, expressing MHETase, we design a new part BBa_K3997005, which is expressing MHETase.
The BBa_K2013003 part is useful as an example. iGEM16_UESTC-China 2016 has cloned this part into pUC57 and did codon optimization before synthesizing it to encode the target product successfully in E. coli.
The difference is that our goal is to build an environmental-friendly method to achieve plastic degradation, and we set up a reaction system to test its property of hydrolysis of MHET. We chose MHETase, to construct our desired engineering strain.
First of all, we constructed a composite part BBa_K3997005, and transformed it into E.coli BL21(DE3). We successfully expressed and purified MHETase protein, and detected the enzyme activity.
References
1. Shosuke Yoshida et al. A bacterium that degrades and assimilates poly(ethylene terephthalate), Science (2016).
2. Harry P Austin. et al. Characterization and engineering of a plastic-degrading aromatic polyesterase, PNAS(2018)
3. Chun-Chi Chen et al. General features to enhance enzymatic activity of poly(ethylene terephthalate) hydrolysis, Nature Catalysis(2021).
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 774
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 153
Illegal PstI site found at 774 - 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 657
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 774
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 774
Illegal NgoMIV site found at 342
Illegal NgoMIV site found at 456
Illegal NgoMIV site found at 912
Illegal AgeI site found at 526 - 1000COMPATIBLE WITH RFC[1000]
| None |