Part:BBa_K782085
10x[TALA] operator_minimal promoter_TALA:NLS:VP16_t2a_BFP
- TALA labels represents TAL effector 1257 from zebrafish experiments (Sander et al., 2011).
- DNA binding sites for individual TAL effectors are indicated with square brackets [ ].
Introduction
Our construct contains TALA binding sites that are cloned upstream of minimal promoter. Downstream of promoter are TALA:VP16 and blue fluorescent protein.
TAL effectors (TALEs) are bacterial plant pathogen transcription factors, that bind to DNA by specifically recognizing one base pair with a single tandem repeat in their DNA-binding domain. A tandem TALE repeat contains 33 to 35 amino acids, where the 12th and 13th amino acid, called a “repeat variable diresidue” (RVD), are responsible for specific interactions with the corresponding base pair (Scholze and Boch, 2011).
VP16 is a 490-amino-acid protein that contains a core domain, which is enriched in acidic residues. The VP16 core contains both structured and unstructured regions. The structured region possesses a seat-like structure in which the seat surface recognizes and binds the TAATGARAT. On its own, however, VP16 does not bind to DNA well. Instead, VP16 is stabilized on the DNA by the unstructured region which interacts with Oct-1, HCF-1 and the DNA in the complex. In this complex, VP16 is able to activate transcription (Wysocka et al., 2003).
To enable monitoring level of expression of TAL activator, we linked it with a fluorescent reporter. BFP is a monomeric fluorescent protein with excitation maximum at 402 nm and emision maximum at 457 nm. TAL activator and fluorescent reporter were connected by an intermediate t2A sequence. This sequence causes the ribosome to skip formation of a peptide bond during protein translation, producing the activator and reporter as separate proteins in equimolar amounts (Garg et al., 2012, Kim et al., 2011, de Felipe et al., 2006).
Figure 1: Schematic representation of the construct.
Characterization
We extensively characterized this construct, since it represents essential part of innovative [http://2012.igem.org/Team:Slovenia/TheSwitchPositiveFeedbackLoopSwitch bistable switch based on a positive feedback loop]. Part contains self-activator, necessary for creating a positive feedback loop that improves the classical toggle switch (Gardner et al., 2000) that can only operate with cooperative DNA binding transcription factors. Addition of the autoloop in this part introduces the required nonlinearity into the system and enables the creation of bistable switches from designed DNA binding elements. This switch represents an extremely powerful tool for synthetic biology as we can use its design to prepare numerous orthogonal designed switches differing in the TAL DNA binding domains and operators, which allows creation of complex designed regulatory circuits.
Figure 2:
Left: Addition of Pristinamycine triggers dissociation of PIP:KRAB from its DNA-binding site. Consequently TALB repressor and TALA activator are transcribed. Activator causes transcription of another pair of TALB:KRAB and TALA:VP16, responsible for the positive feedback loop. Meanwhile TALB:KRAB represses the other state of the switch.
Right: When the inducer is removed, the inducible repressor binds back to its DNA-binding site, but since TALA:VP16 is still present, auto-activation is achieved, resulting in a stable state.
Figure 3:
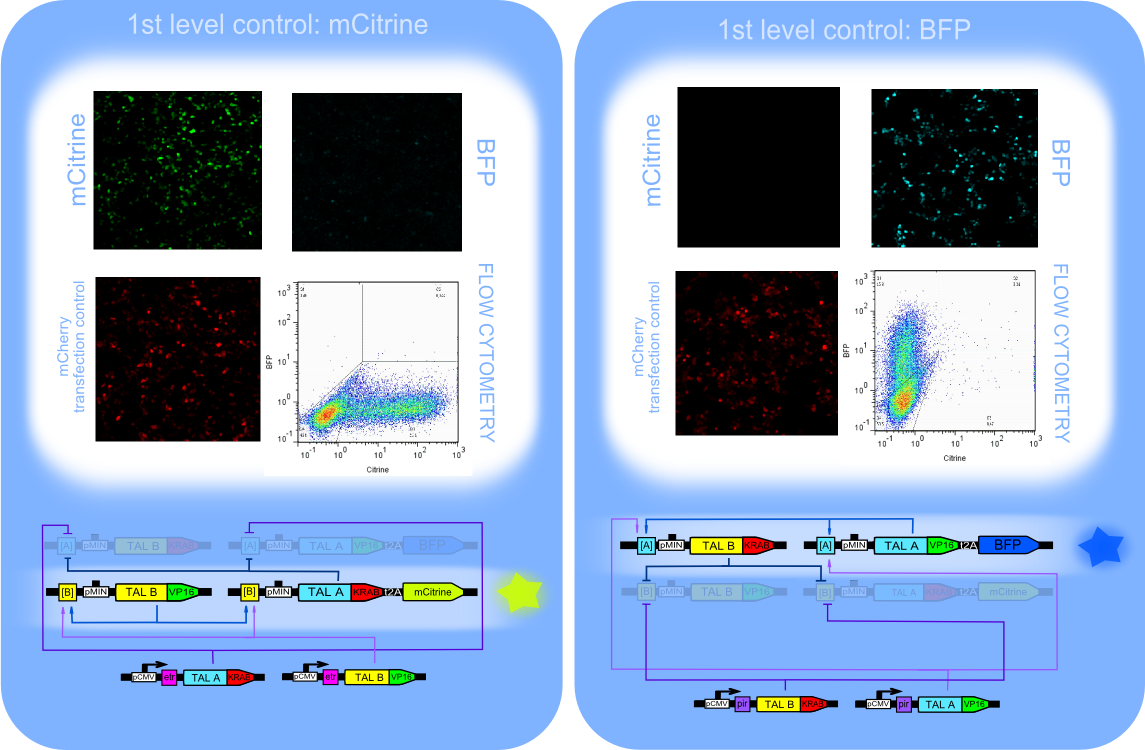
The first level control consisting of four positive feedback loop switch components ([A]_PMIN_TALB:KRAB, [A]_PMIN_TALA:VP16_t2A_BFP, [B]_PMIN_TALA:KRAB_t2A_mCitrine, [B]_PMIN_TALB:VP16)
Left: TALA:KRAB and TALB:VP16 are constantly expressed by CMV promoter and influence on the other parts of the switch. mCitrine is activated, since TALB:VP16 is expressed while BFP is repressed becouse of TALA:KRAB. Right:TALB:KRAB and TALA:VP16 are constantly expressed by CMV promoter. Activator activates constructs under A binding sites, meanwhile repressor represses the other state of the switch.
Figure 4:
Second level control is composed form all components, missing only one of the inducible repressors. Since only one inducible repressor is present, it binds to it's binding sites and inactivates transcription. Therefore all parts that are regulated by the same binding sites are repressed, meaning one pair of inducible constructs are active and turn the switch in one state.
Figure 5:
Four positive feedback loop switch components ([A]_PMIN_TALB:KRAB, [A]_PMIN_TALA:VP16_t2A_BFP, [B]_PMIN_TALA:KRAB_t2A_mCitrine, [B]_PMIN_TALB:VP16) mixed with constantly expressed TAL:KRAB and TAL:VP16. Results show that upon addition of different TAL regulators the switch shifts in one state.
References
Gardner, T. S., Cantor, C. R. and Collins, J. J. (2000) Construction of a genetic toggle switch in Escherichia coli. Nature. 403, 339–342.
Sander, J. D., Cade, L., Khayter, C., Reyon, D., Peterson, R. T., Joung, J. K., and Yeh, J.-R. J. (2011) Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nature Biotechnology 29, 697–698.
Scholze, H., and Boch, J. (2011) TAL effectors are remote controls for gene activation. Curr. Opin. Microbiol. 14, 47-53.
Kim, J. H., Lee, S.-R., Li, L.-H., Park, H.-J., Park, J.-H., Lee, K. Y., Kim, M.-K., et al. 2011. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PloS one 6,e18556.
de Felipe, P., Luke, G. a, Hughes, L. E., Gani, D., Halpin, C., Ryan, M. D. 2006. E unum pluribus: multiple proteins from a self-processing polyprotein. Trends in biotechnology 24,68–75.
Wysocka J. and Herr W (2003) The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. TRENDS in Biochemical Sciences 28, 294-304.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 750
Illegal BamHI site found at 720
Illegal BamHI site found at 3262
Illegal XhoI site found at 788 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 147
Illegal NgoMIV site found at 507
Illegal AgeI site found at 12
Illegal AgeI site found at 347
Illegal AgeI site found at 372
Illegal AgeI site found at 707 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 4207
| None |