Part:BBa_K581019
rhl repressible promoter-gfp-ssrA degradation tag
We selected an AHL-based quorum-sensing system, RhlR-RhlI pair from Pseudomonas aeruginosa as a proof of concept to design quorum sensing repressible promoter. RhlI is a synthase that produces signal molecule butanoyl (C4-HSL) and RhlR is the regulator that responds to C4-HSL and is supposed to activate transcription at cognate rhl promoter. Genetic evidence suggests that RhlR binds to conserved sequence of some quorum-controlled promoters, what is called rhl box.
We selected rhl-box in the promoter region of rhl-specific gene rhlA, to which LasR (another transcriptional regulator in Pseudomonas aeruginosa) doesn’t bind, and then created the rhl repressible promoter by positioning the rhl-box between and partially overlapping consensus -35 and -10 hexamers with 18 bp between the hexamers. When C4-HSL represents and binds to RhlR proteins, RhlR will bind to its DNA binding site, the rhl box, thus to reduce the accessibility of RNA polymerases to this promoter, repressing the transcription initiation.
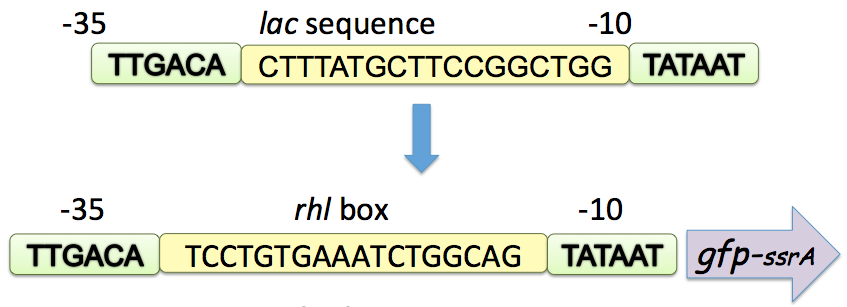
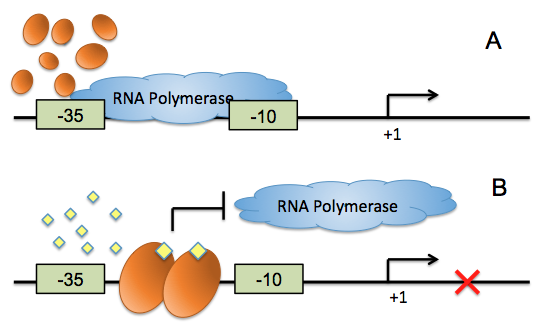
Figure 2: The mechanism of AHL repressible promoter. (A) RNA polymerase functions well when AHL signal is absent. (B) When AHL presents and binds to transcriptional regulator, the transcription regulator binds its DNA binding site, thus to reduce the accessibility of RNA polymerase to promoter, repressing the transcription initiation.
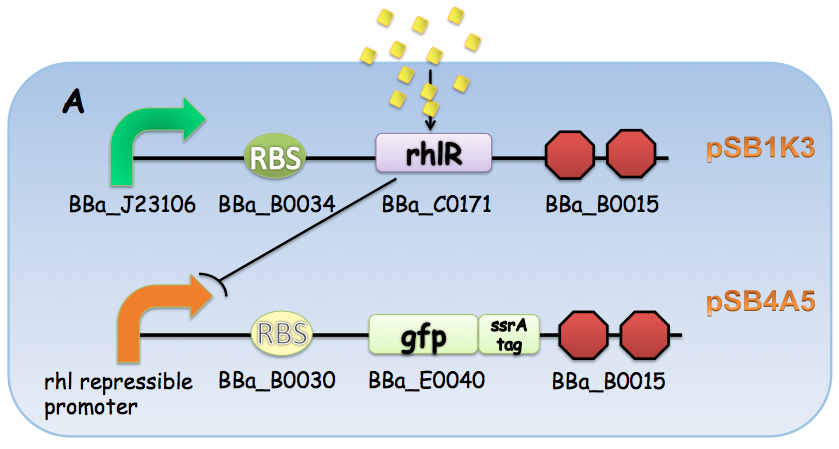
A GFP reporter with C-terminus fused ssrA degredation tag was used to report the transcriptional activity at the promoters. The rhl-box promoter-GFP ssrA was then cloned into pSB4A5 backbone and the BBa_J23106 (constitutive promoter)-rhlR- BBa_B0015 (terminator) into pSB1K3.
To evaluate the properties of rhl repressible promoter, we applied dose response assay and time dependence assay. In these assays GFP intensity was measured to quatitatively evaluate the promoter acitivity by Tecan Microplate Reader with excitation wavelength at 470nm and emission wavelength at 509nm. A black 96-well plate was used to minimize the interference of different well. OD 600 was also measured by Tecan Microplate Reader in a transparent 96-well plate.
Dose response assay
An overnight culture of bacteria grown in LB with ampicillin and kanamycin at 37°C was reactivated by diluting the culture in a ratio of 1:1000 with fresh LB. The LB we used was pre-mixed with different dose of C4-HSL and its final concentration varied from 0 to 1mM/1μM. When OD600 reached 0.6-0.8, pellet bacterial cells by 4 min centrifugation at 4000 rpm, discard the supernatant. Resuspend the pelleted cells in 500 μl of PBS, and then pippete 200uL of bacterial resuspention into each well of 96-well plate.
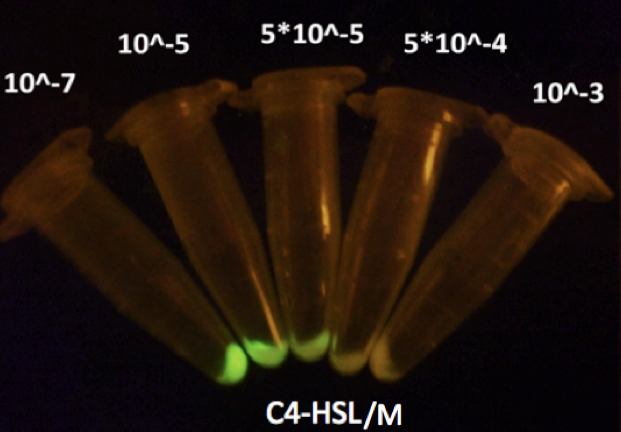
Figure 4 shows repression of GFP expression in circuits depicted in Fig 2 under gradient concentrations of C4-HSL, which indicates that the activity of rhl repressible promoter was C4-HSL dependent, with strongest repression at 5*10^-4 M of C4-HSL.
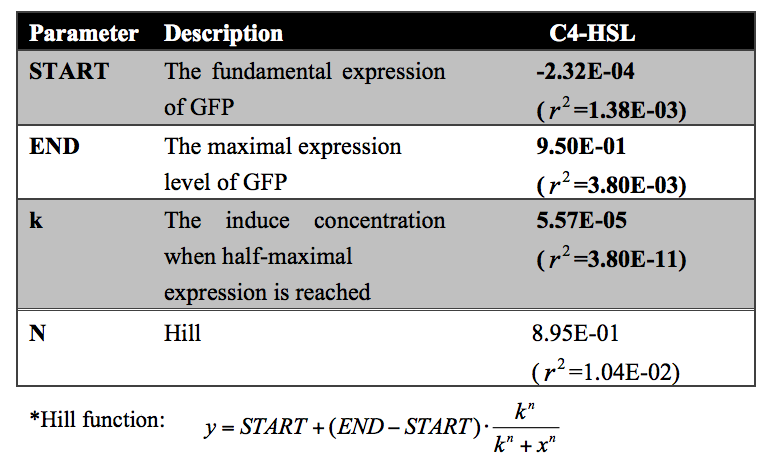
Quantitative analysis was also conducted. As shown in the following figure and table, the dose response curve represents as a hill function and the decrease of transcription activity at promoter was approximate 20-fold.
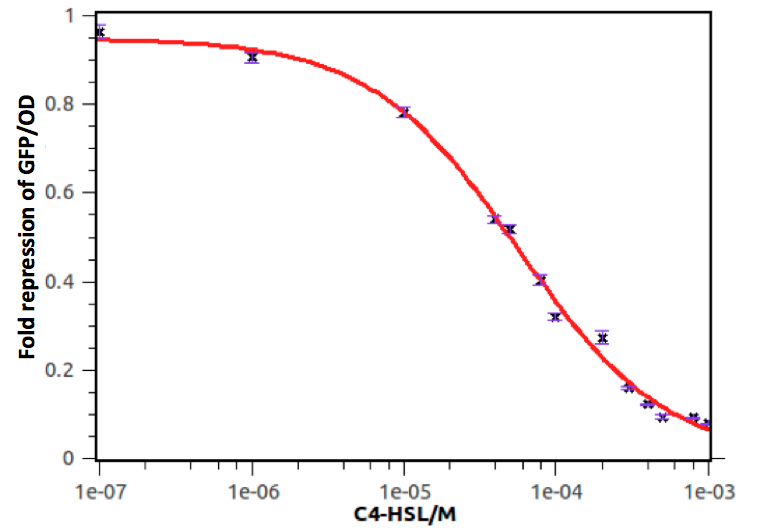
Figure 5: Fold repression of GFP/OD expressed by rhl repressible promoter on C4-HSL signal concentrations. In this experiment, GFP expression in E. coli containing pSB1K3 and pSB4A5 was measured at a series of concentrations of C4-HSL. The fold induction of GFP/OD is expressed as the percentage of expression in the absence of C4-HSL. Error bars correspond to the standard deviation from multiple measurements.
Therefore, we have demonstrated that the AHL repressible promoter with rhl-box owes the ability to convert transcriptional activator rhlR into repressor.
Time dependence assay
An overnight culture of grown in LB with ampicillin and kanamycin at 37°C was reactivated by diluting the culture in a ratio of 1:1000 with fresh LB. When OD600 reached 0.4, the bacteria was disposed to several EP tubes, each owning 500uL, and C4-HSL was supplied with 3 duplicates and the final concentration was 1mM. We cultured the fluorescence in EP tubes with 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8 hours at 37°C. Then pellet bacterial cells by 4 min centrifugation at 4000 rpm, discard the supernatant. Resuspend the pelleted cells in 500 μl of PBS, and then pippete 200uL of bacterial resuspention into each well of 96-well plate.
The results of the assay are displayed below.
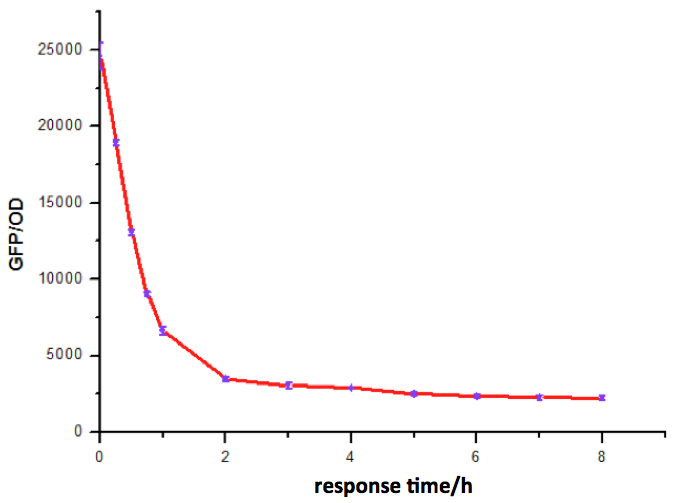
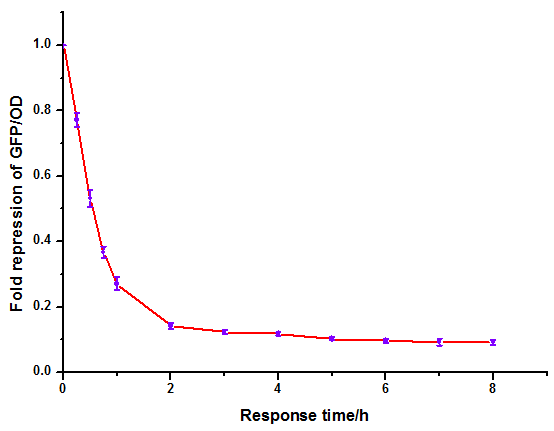
Figure 6: Time dependence of AHL mediated repression at rhl repressible promoter under 1mM of C4-HSL . (A) GFP/OD; (B) fold repression of GFP/OD. Error bars correspond to the standard deviation from multiple measurements.
Noting that the GFP expression intensity was reduced from 100% to 10% in merely 2-3 hours, we were delighted by the dramatic repression performance of quorum sensing repressors. The time dependence assay provides further promising clues towards the expansion of our quorum sensing repressor design to more QS systems.
Reference
[1] CA Voigt. Genetic parts to program bacteria. Curr Opin Biotechnol 17, 548–557 (2006).
[2] K Clancy, CA Voigt. Programming cells: towards an automated 'Genetic Compiler'. Curr Opin Biotechnol 21, 572–581 (2010) .
[3] KA Egland, EP Greenberg. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J. Bacteriol 182, 805-811 (2000).
[4] M Schuster, ML Urbanowski, EP Greenberg. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. PNAS 45, 15833-15839 (2004).
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal SpeI site found at 808
- 12INCOMPATIBLE WITH RFC[12]Illegal SpeI site found at 808
- 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal SpeI site found at 808
- 25INCOMPATIBLE WITH RFC[25]Illegal SpeI site found at 808
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 703
| None |
