Part:BBa_K554005
IL12
Contribution by 2022 iGEM Team Shanghai_HS
BBa_K4276001
Name: IL12
Base Pairs: 1593 bp
Origin: synthesis
Properties: pro-inflammatory type-1 cytokine
Usage and biology
Interleukin-12 (IL-12) is a pro-inflammatory type-1 cytokine which bridges the innate resistance and adaptor immunity. Also, IL-12 has long been studied as a potential vaccine adjuvant to stimulate the immune. In a study reported that oral administration of IL-12 could induce immune responses.
Experiment data
1.2 PCR cloning IL-12 DNA fragment
The IL-12 DNA fragments PCR result showed in Figure 2. Compared to DNA maker, the PCR products exhibits the correct bands.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1409
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
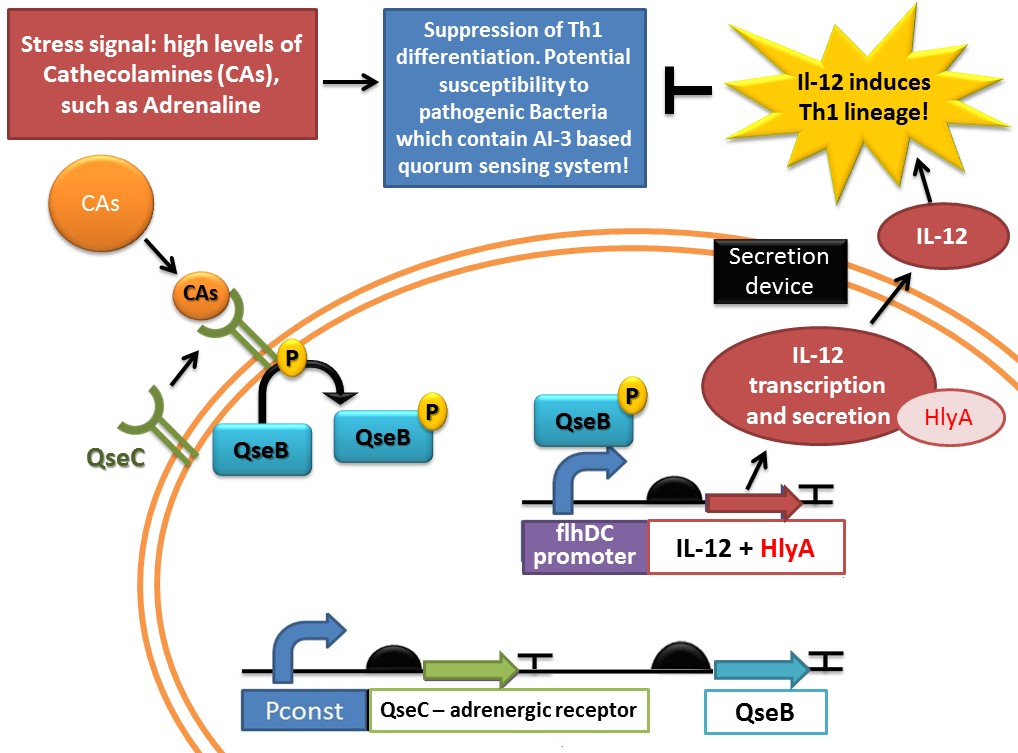
IL12 is an heterodimeric cytokine, composed of a disulfide-bound 35 kDa subunit (p35) and a 40 kDa subunit (p40) which enhances both innate and adaptative immune system. It is primarily produced by antigen-presenting cells such as dentritic cells.MORALES et al. (2004) established that sustained IL-12 signaling is required for Th1 development: It drives naïve Th0 differentiation towards the Th1 phenotype, which makes it an appropriate answer to a Th2 imbalance. We thus decided to create a completely new biobrick, responsible for Il-12 synthesis. The IL-12 gene is used by [http://2011.igem.org/Team:UNICAMP-EMSE_Brazil UNICAMP-EMSE Brazil team] in the [http://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_1:_Adrenaline_sensor.2FIL-12_producer CA sensor device/ IL-12 producer] ("Device 1", which senses Catecholamine levels (such as Adrenaline, Norepinephrine and AI-3) and responds by producing and secreting IL-12).
A fusion IL-12 protein
- As MORRIS et al. (2008) [4] recollect, “Early approaches in generating recombinant IL-12 involved co-transfection of cells with two different vectors, each expressing one of the two genes (p40 or p35), or expression of the individual genes under the control of separate promoters in a single plasmid or viral vector. Both approaches were disadvantaged due to inefficient expression of the functional heterodimer with the likelihood of p40 homodimer production” (And thus competitive IL-12 antagonist action.)
- In order to counter those difficulties, several research teams reported the expression of a bioactive single chain porcine IL-12 fusion protein, such as LIESCHKE et al. (1997), whose protein comprised the p40 subunit linked by a (Gly, Ser) linker to the p35 subunit from which the first 22 amino acids were deleted. It proved bioactive with an apparent specific activity comparable to recombinant human IL-12.
Our IL-12 sequence
- As successful work (correct synthesis and protein proved bioactive) has already been done on it , we chose to use a fusion construct of human IL-12, similarly as the one used by LIESCHKE et al. (1997) porcine fusion IL-12 protein for our project.
Three-dimensional structure representation
You can find below a tridimensional structure of human p70 Interleukin 12 (retrieved from PDB 1F45 (Yoon et al. 2000)), with both p35 (chain A) and p40 (chain B) chains. Yoon et al (2000) solved the crystal structures of monomeric human p40 at 2.5 A and the human p70 complex at 2.8 A resolution, which reveals that IL-12 is similar to class 1 cytokine-receptor complexes. This is a jmol applet, in which you can interactively see the protein structure:
A synthesis trigger: promoter flhDC
- We need a device that only works when needed: recombinant IL-12 expression must not be continuous, it has to be a reaction to a Th2 imbalance and stop when the balance is reestablished. Therefore, we need a promoter that would be a sensible “trigger”.
- As we explained in the sensor part (the bacterial adrenergic sensor), our device will detect a Th2 imbalance via QseB/QseC sensor, that will be activated in response to adrenaline detection. It will then be able to activate the flhDC promoter (SPERANDIO et al. , 2002) that we will be used to control the IL-12 gene expression.
IL-12 secretion
- With this right promoter, a ribosome binding site (RBS), the IL-12 gene, and a terminator, E.Coli is now able to synthesize IL-12 in response to a Th2 imbalance. But, being a bacteria, it doesn’t have the required material to secrete it in the body. Hence we also need to construct a secretion system, that will be described in the dedicated part of this wiki.
- In our “IL-12 synthesis part”, we will just add a secretion signal sequence (HlyA) for IL-12 to be correctly dealt with by the secretion system.
Schema:
This sequence is used by [http://2011.igem.org/Team:UNICAMP-EMSE_Brazil UNICAMP-EMSE Brazil team] in the [http://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project/Device1 Adrenaline sensor device] ("Device 1", which senses Cathecolamine and AI-3 levels and responds by producing and secreting IL-12. This part is shown in red in the following schema:
References
- Vernonica Athie-Morales et al, Sustained IL-12 Signaling Is Required for TH1 Development, JI, 2004, 172 [http://www.ncbi.nlm.nih.gov/pubmed/14688310 Link to PubMed]
- K. R. Morris et al, Expression of biologically active recombinant porcine interleukin-12 from Escherichia coli, Veterinary Immunology and Immunopathology, 2008, 126 [http://www.ncbi.nlm.nih.gov/pubmed/18823664 Link to PubMed]
- Graham J. Lieschke et al, Bioactive murine and human interleukine-12 fusion proteins which retain antitumor activity in vivo, Nature Biotechnology, 1997, Vol 15. [http://www.nature.com/nbt/journal/v15/n1/abs/nbt0197-35.html Article link]
- Yoon, C., Johnston, S.C., Tang, J., Stahl, M., Tobin, J.F., Somers, W.S. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. (2000) EMBO J. 19: 3530-3541 [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=10899108 Link to PubMed]
- Sperandio V, Torres AG, Kaper JB (2002) Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43: 809–821. [http://www.ncbi.nlm.nih.gov/pubmed/11929534 Link to PubMed]
| None |