Part:BBa_K551001
plasmid of insertion and deletion
The pINDEL plasmid is composed of 2 functional units:
- the IN function which is composed of the gam, exo and bet genes coding for the lambda Red recombinase system
- the DEL function which is based on the flp gene encoding the FLP site-specific recombinase and expressed at 42┬░C
Another feature of pINDEL is that its replication origin is not functional at 42┬░C due to the thermosensitivity of the RepA101Ts protein. This protein initiates replication at the ori site in permissive conditions (30┬░C) and is inactive at 42┬░C.
The deletion function and the thermosensitivity of the plasmid both could be shown to be working as intended (see functional parameters). The insertion function couldn't be properly tested.
Usage and Biology
The use of pINDEL is quite easy, you can check our visual how to manual here Media:Pindel-How-To.pdf
Basically you need to assemble BBa_K551000 with your biobrick of choice through the standard assembly. While your assembly proceeds, you order two primers to amplify this new assembled biobrick. The trick here is to add 40 nucleotides on the 5' end of each primer; these 40 nucleotides need to be homologous to the desired point of insertion. A good target is the lacZ gene as it allows easy white/blue check for insertion in addition of the resistance marker.
Once you have your biobrick of choice assembled with BBa_K551000, proceed with your PCR; all the while you transform your E.coli strain of choice with pINDEL and prepare those as [http://openwetware.org/wiki/Electrocompetent_cells electrocompetent cells] it is essential to grow them with arabinose to allow the RED recombinase system to be expressed ! You electroporate the PCR fragment (ensure that the template plasmid isn't AmpR or CmR otherwise first [http://openwetware.org/images/4/4b/SEED_gel_purification.pdf purify the fragment]) and then incubate your plates (with Amp and Cm) at 30┬░C (don't forget pINDEL is thermosensitive). The recombination should happen overnight, but at 30┬░C it may take a couple of days for your colonies to be visible, if you need an illustration of homologous recombination : Media:Homologous-pINDEL.pdfŌĆÄ
For the resistance gene excision, you streak the colonies on LB plates and incubate them over night at 42┬░C, this will express the Flipase and remove pINDEL from your bacteria. Restreak candidates on LB, on LB+Amp to control the loss of pINDEL and on LB+Cm to control the resistance removal. Incubate over nigth at 37┬░C et voil├Ā ! You now have a clean, resistance free chromosomal insertion.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 396
Illegal EcoRI site found at 3708
Illegal EcoRI site found at 5217
Illegal EcoRI site found at 6322
Illegal XbaI site found at 423
Illegal SpeI site found at 6004
Illegal SpeI site found at 8956
Illegal PstI site found at 435
Illegal PstI site found at 4712
Illegal PstI site found at 4959 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 396
Illegal EcoRI site found at 3708
Illegal EcoRI site found at 5217
Illegal EcoRI site found at 6322
Illegal SpeI site found at 6004
Illegal SpeI site found at 8956
Illegal PstI site found at 435
Illegal PstI site found at 4712
Illegal PstI site found at 4959
Illegal NotI site found at 9275 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 396
Illegal EcoRI site found at 3708
Illegal EcoRI site found at 5217
Illegal EcoRI site found at 6322
Illegal BglII site found at 632
Illegal BglII site found at 1728
Illegal BamHI site found at 417
Illegal BamHI site found at 3647 - 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 396
Illegal EcoRI site found at 3708
Illegal EcoRI site found at 5217
Illegal EcoRI site found at 6322
Illegal XbaI site found at 423
Illegal SpeI site found at 6004
Illegal SpeI site found at 8956
Illegal PstI site found at 435
Illegal PstI site found at 4712
Illegal PstI site found at 4959 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 396
Illegal EcoRI site found at 3708
Illegal EcoRI site found at 5217
Illegal EcoRI site found at 6322
Illegal XbaI site found at 423
Illegal SpeI site found at 6004
Illegal SpeI site found at 8956
Illegal PstI site found at 435
Illegal PstI site found at 4712
Illegal PstI site found at 4959
Illegal AgeI site found at 3482
Illegal AgeI site found at 5139 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 837
Illegal BsaI.rc site found at 9958
Illegal SapI site found at 989
Illegal SapI site found at 1787
Illegal SapI site found at 3464
Functional Parameters
Thermosensitivity
We performed a thermal sensitivity experiment in liquid medium in which we measured the plasmid loss frequency as a function of time of culture at 42┬░C. We compared the viability (CFU/ml: colony forming units per ml of culture) of MC1061/pIN grown at 30┬░C and 42┬░C and plated on LB and LB Amp medium. If the plasmid is lost, then the bacteria won't grow on the LB Amp medium plates.
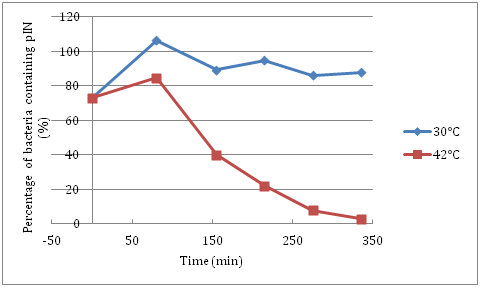
This shows that the replication of pIN is impaired at 42┬░C. After 150 min of culture at 42┬░C, 60% of the bacteria have lost the pIN plasmid. After 275 min, only a very small proportion of bacteria retained the pIN plasmid (< 10%). Note that at time 0 of the experiment, around 70% of the bacteria retained the pIN plasmid suggesting that the repA101ts origin is not fully active even at 30┬░C. With time, the pIN plasmid is maintained in about 80% of the bacteria at 30┬░C. This experiment was performed only once due to time limitations and should be repeated to confirm the data.
Deletion function
To test the deletion capacity of the pINDEL plasmid, we transformed pINDEL and pIN in the MG1655 ŌłåtldD ::frt-cm-frt strain and the transformants were selected on LB Amp Cm medium at 30┬░C. Four independant colonies containing pIN or pINDEL were streaked on LB plates and were incubated ON at 42┬░C to lose plasmids and to express the flipase. For each independant transformants streaked we stabbed 8 colonies on LB and LB Cm plates.
All 32 candidates that contained pIN grew on LB plates and LB Cm plates indicating that none of them has lost the antibiotic resistance cassette. All 32 candidates that contained pINDEL grew on LB but none of them grew on LB Cm plates indicating that all of them have lost the antibiotic resistance cassette.
This gives us a 100% success rate for excision.
Growth
In order to test if the leak of promoter ╬╗pR of flippase or the expression of Red genes affects bacterial growth we measured OD600nm of growing MC1061 strain containing pIN or pINDEL.
These bacteria were cultivated overnight at 30┬░C in LB and in LB with 1% arabinose. The overnight cultures were diluted at an OD600nm of 0.01 in the same medium and incubated at 30┬░C with shaking. The OD600nm was measured every 30 minutes.
This shows that strains containing the pIN plasmid or the pINDEL plasmid have more or less the same growth rate in the same medium indicating that the leak of the flippase promoter has few (or no) effect on bacterial growth.
However when arabinose was added to the medium growth rate of both strains is affected indicating that Red genes expression has a detrimental effect on bacterial growth.
| resistance | 100 Ąg/ml |

