Part:BBa_K4968008
CsgA-AG4
The composite part consists of CsgA (BBa_K4968004) and AG4 (BBa_K4968005) will be introduced into two strains, BL21(DE3) and MC4100, both with genomic genes CsgA and CsgB knockout. In E.coli, CsgA protein self-assembles into amyloid nanofibers, or curli fibers. AG4, a synthetic silver-binding dodecapeptide. The experimenters combined it with a curli fiber membrane formed by CsgA protein to improve the efficiency of adsorbing silver ions in sewage.
Figure 1 | The prediction of the structure of CsgA-AG4 fusion protein by alpha-fold. The alpha-fold picture of the fusion protein. From the figure, the fusion protein has many β sheets, which belong to amyloid. The part of the protein that sticks out is AG4. Outside the outer membrane, the CsgA-AG4 will self-assemble.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 198
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 58
- 1000COMPATIBLE WITH RFC[1000]
Usage & biology
A section of the curli operon, CsgA, and CsgB gene, has been removed from the genomes of BL21(DE3) and MC4100 strains. The CsgB gene anchors curli fiber proteins formed by CsgA to the bacterial cell's outer membrane, which needs to be knocked out to facilitate curli fiber secretion into the extracellular matrix (Courchesne et al., 2016). In the presence of CsgB, CsgA polymerizes intracellularly (Hammer et al., 2007). However, in the absence of CsgB, CsgA can also aggregate extracellularly (Wang et al., 2008). The expression of the target fusion protein CsgA-AG4 entirely relies on the exogenously introduced plasmid, as the genomic CsgA and CsgB gene has been knocked out in the strain's genome.
CsgA is the critical protein component of curli fibers assembled by intestinal bacteria like Escherichia coli and Salmonella spp. (Wang and Chapman, 2008). In the absence of CsgB, CsgA can self-assemble into amyloid nanofibers extracellularly, which can be filtered by a vacuum filter with a 10μm pore size polycarbonate membrane. In this context, AG4 serves as a protein with potential applications in adsorbing valuable silver ions from the environment (Seker and Demir, 2011). CsgA has been shown to bind to small molecular weight proteins like mussel foot proteins (Mfps), helping maintain their function and facilitating extracellular secretion (Zhong et al., 2014). Consequently, the experimenters aim to combine CsgA and AG4 proteins to construct an independent nanofiber that can absorb silver ions in sewage.
Both CsgA and AG4 have undergone codon optimization, which offers several benefits. Codon optimization enhances protein expression efficiency in the host organism, potentially improving the yield of the fusion protein CsgA-AG4. It ensures that the codons used are better suited to the host's translational machinery, reducing the risk of translation errors or protein misfolding. Additionally, codon optimization can enhance the overall stability of the expressed protein, increasing its functionality and potential applications in adsorbing silver ions from the environment.
The primary purpose of this structure is to express and secrete the fusion protein CsgA-AG4 and build the nanofiber on the 10μm pore size polycarbonate membrane. After protein expression, it is filtered with a vacuum filter and a 10μm pore size polycarbonate membrane is used to host the fusion protein. The polycarbonate membrane then should be stained by Congo red dye as below.
Figure 2 | Congo red staining of polycarbonate membrane with CsgA-Ag4 fusion protein. This figure demonstrates the expression of the fusion protein CsgA-AG4 in BL21(DE3) [ΔCsgA & ΔCsgB] and MC4100 [ΔCsgA & ΔCsgB] strains following purification and filtration through a 10μm pore size polycarbonate membrane. This confirms the successful expression of CsgA, a type of amyloid.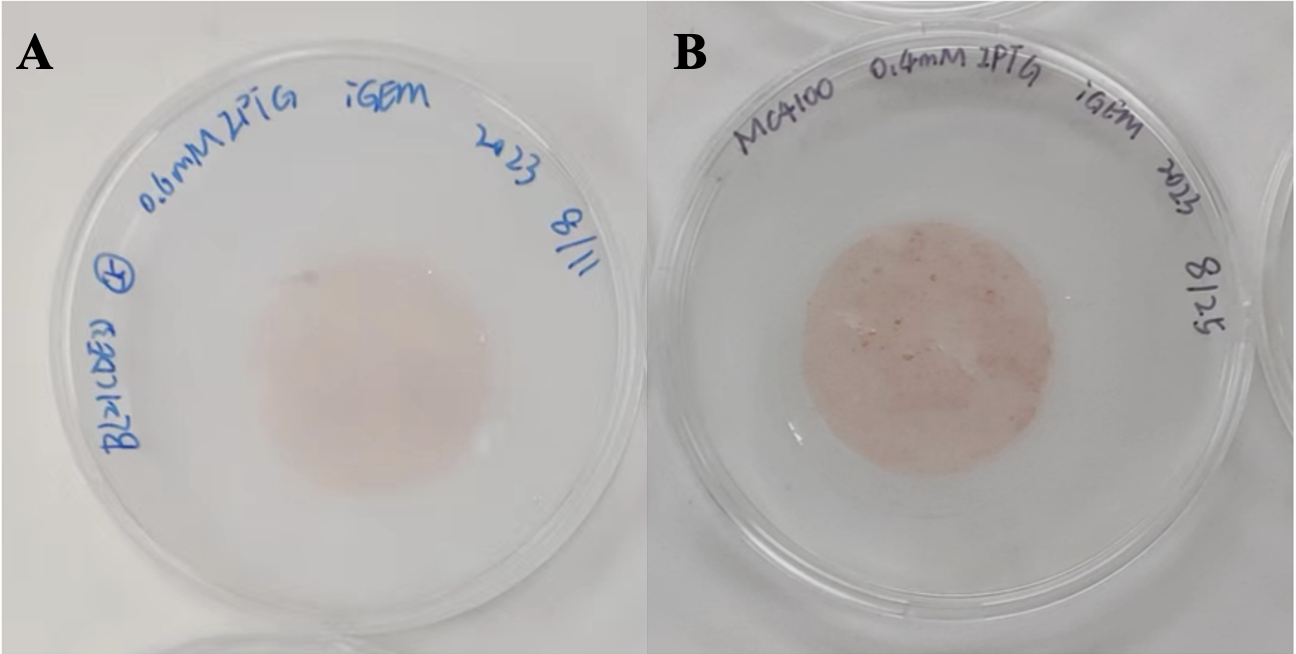
To further verify the successful expression of this composite part, Scanning Electron Microscopy (SEM) is used to image the specific curli fibers.
Figure 3 | Scanning Electron Microscopy (SEM) analysis. The experimenter used SEM to observe the fusion protein produced by the MC4100 strain. The part marked in red in the figure is the CsgA-AG4 fusion protein which can be seen clearly. The CsgA-AG4 fusion protein was observed on the 10 μm polycarbonate filter film. The overlap of the CsgA-AG4 fusion protein indicated the successful expression. The magnification of A, B, and C are 5 KX, 10 KX, and 20 KX respectively (done by Yuantest Laboratory).
Moreover, the enhanced adsorption of silver ions further augments the functionality of AG4, opening up potential applications in metal ion removal and environmental remediation. To validate the silver ion-binding capability of AG4 peptide, we developed a model using a fusion protein membrane for silver ion adsorption. To assess the membrane's efficacy, we conducted evaluations based on three parameters: time, temperature, and silver ion concentration. Our results conclusively demonstrated AG4's exceptional silver ion adsorption capacity. This system holds promise as a valuable tool for combating environmental pollution and recovering valuable metals from industrial processes.
The experimenters used ICP-MS to test the ability of adsorbing silver ions as follows.
Figure 4 | Assessment of optimal temperature. This graph indicates the adsorption efficiency of CsgA-AG4 recombinant protein from three strains at various temperatures over an 8-hour period. The optimal temperature is found to be 25℃. The fusion protein CsgA-AG4 of all three strains have the significant difference compared with the control group. Figure 5 | Assessment of optimal time. This graph depicts the adsorption efficiency of CsgA-AG4 recombinant protein from three strains at 25°C across varying adsorption times. The figure highlights the optimal adsorption time is found to be 8 hours. The fusion protein CsgA-AG4 of all three strains have the significant difference compared with the control group. Figure 6 | Assessment of optimal silver ion concentration. This graph illustrates the adsorption efficiency of CsgA-AG4 recombinant protein from three strains at 25℃ across varying silver ion concentrations during an 8-hour incubation period. The fusion protein CsgA-AG4 of all three strains have the significant difference compared with the control group. Figure 7 | The function image of time-temperature-adsorption efficiency. A represents a three-dimensional graph illustrating the adsorption efficiency as a function of temperature and time, obtained through fitting. The face center composite design (α=1) was carried out using the design expert 13. B displays a contour plot of the same function. C and D showcase interactions between temperature and time. The resulting graph is as above. 8 hours and 25℃ are proper for proteins to absorb silver ions.



Additionally, in order to further test the functionality of the fusion protein, Scanning Transmission Electron Microscopy (STEM) was used to detect the content of adsorbed silver ions and the proportions of each element in the protein.
Figure 8 | SEM electron microscopy shows the image and the percentage of each element in the protein.The experimenters selected the central region of the whole protein for elemental content analysis. This allowed for the intuitively visual observation of the distribution and content of various elements within the target protein. The presence of silver elements can demonstrate the absorption of silver by the target protein (done by Yuantest Laboratory). Figure 9 | SEM Elemental Mapping of CsgA-AG4 after absorbing silver ions. (a) Element carbon distribution (red). (b) Element nitrogen distribution (blue). (c) Element oxygen distribution (purple). (d) Element sulfur distribution (dark green). (e) Element silver distribution (yellow). (f) Element phosphorus distribution (light green). Scale bar: 1μm (done by Yuantest Laboratory). Figure 10 | STEM-HAADF imaging of nano silver synthesis on CsgA-AG4 fusion protein (30kV). The milky white material in ABEF in the picture is silver element and the black lumpy material in CDG in the picture is silver element. This also shows that the CsgA-AG4 fusion protein actually adsorbs silver ions. The magnification of A~G are 10.00KX, 20.00KX, 10.00KX, 20.00KX, 10.00KX, 50.00KX, 50.00KX (done by Yuantest Laboratory). 


In summary, the CsgA-AG4 plasmid is a versatile genetic tool that can synthesize curli protein fibers, construct independent curli fiber membranes, and express AG4. It could be an essential tool for capturing silver ions in sewage in the future. The plasmid's design accommodates the genetic modification needs of MC4100 and BL21(DE3) strains and enables normal expression in the host. It offers the potential for future applications in environmental issues and basic research.
Source
The sequence of CsgA is from NCBI. The sequence of AG4 is from the literature “Solution structure of peptide AG4 used to form silver nanoparticles” (Asn1-Pro-Ser-Ser-Leu-Phe-Arg-Tyr-Leu-Pro-Ser-Asp)
References
Hammer, N. D., Schmidt, J. C., Chapman, M. R. (2007) ‘The curli nucleator protein, CsgB, contains anamyloidogenic domain that directs CsgA polymerization’ Proc. Natl. Acad. Sci. U. S. A. 104 (30)12494- 12499. Available at: https://doi:10.1073/pnas.0703310104
Seker, U.O.S. and Demir, H.V. (2011) ‘Material Binding Peptides for Nanotechnolgy’ Molecules 16(2),1426-1451. Available at: https://doi.org/10.3390/molecules16021426
Wang, X., Hammer, N. D., Chapman, M. R. (2008) ‘The Molecular Basis of Functional Bacterial AmyloidPolymerization and Nucleation’ J. Biol. Chem. 283 (31) 21530- 21539. Available at: https://doi:10.1074/jbc.M800466200
Wang, X. and Chapman, M.R. (2008) ‘Sequence Determinants of BacterialAmyloid Formation’ Journal of Molecular Biology 380 (3) 570-580. Available at: https://doi.org/10.1016/j.jmb.2008.05.019
Zhong, C. et al. (2014) ‘Strong underwater adhesives made by selfassembling multi-protein nanofibres’ nature nanotechnology 9, 858-866 (2014). Available at: https://doi.org/10.1038/nnano.2014.199
| None |
