Part:BBa_K4275028
eforRED-dockerin I
This composite part is consisted of a type i dockerin and a eforRED chromoprotein fused at the N terminal of the dockerin. The dockerin is a highly symmetrical and non-catalytic domain and it specifically binds with type i cohesin on the scaffoldin by high-affinity and non-covalent interactions. In cellulosome, the type one dockerins are usually fused on enzymes thus enabling the assembly of various cellulase on the CipA scaffoldins to ensure enzymatic synergies. The eforRED protein (originates from Echinopora forskaliana ) [2] fused at the N terminal of the dockerin is a reporter molecule, serving the purpose of testing whether the cohesin and the dockerin are successfully bind. By centrifugation, the successfully combined complex will display a red fluorescent.
Figure 1 The 3D structure of the protein predicted by Alphafold2.
Usage and Biology
Type i dockerin is a module which anchors the catalytic subunits to the scaffoldins. It is originally found in anaerobic bacteria C.thermocellum[1]. Its sequence is a tandem duplication of a 22-residue segment and it displays internal two-fold symmetry, consisting of a duplicated F-hand motif (a calcium-binding loop preceding an alpha helix)[1]. As a result, it requires calcium ions to be presented due to the the calcium-binding motif of the dockerin domain whose sequence resembles the EF-hand motif of calcium-binding proteins. The binding of type i dockerin and cohesin also highly relies on the polar interactions between Ser-45 and Thr-46[1].
eforRED as A Reporter Protein
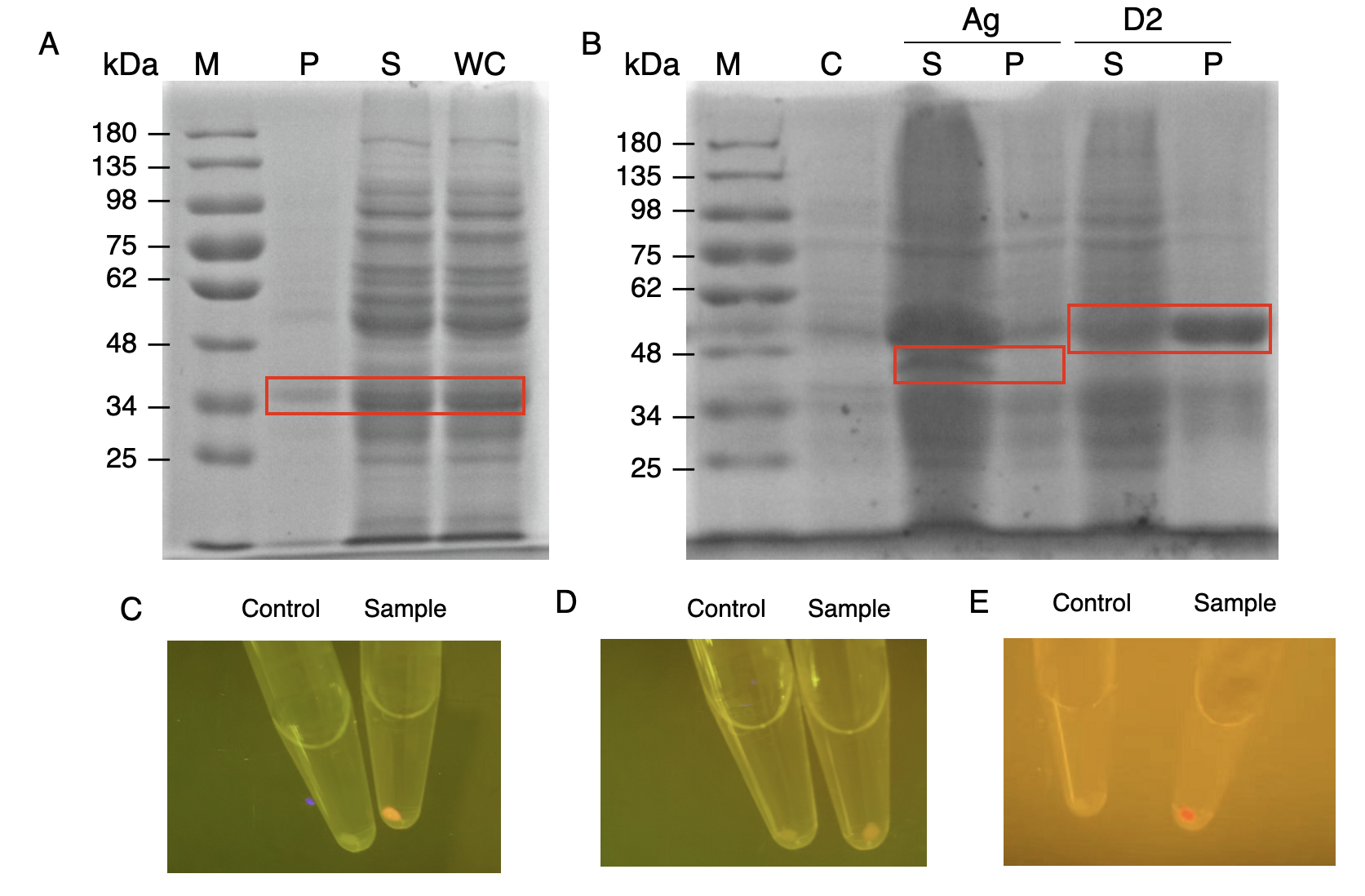
The nanobody-antigen interaction was verified by mixing intact E.coli cells displaying Neae-Nb3 with the supernatant of Ag3-eforRED (Fig. 2A). Red fluorescent characteristics were observed in the pellets after resuspending the centrifuged mixture, which is absent in the control group that only contains Neae-Nb3 (Fig. 3C).
After that, the type II cohesin-dockerin interaction was tested using the mixture of Neae-Nb3, OlpB-Ag3, and the type II dockerin fused with eforRED (Fig. 2B). A negative control lacking OlpB-Ag3 was set up for result comparison. Centrifugation was used to remove supernatant and the red fluorescence was only identified in pellets of the sample group, confirming the type II cohesin-dockerin interaction (Fig. 3D).
Finally, the association between type I cohesin and type I dockerin was validated using the mixture of Neae-Nb3, OlpB-Ag3, CipA1B2C, and DocI-eforRED (Fig. 2C), red fluorescence was detected in the resuspended mixture while it was not observed in the control group lacking the primary scaffold CipA1B2C (Fig. 3E), verifying the type I cohesin-dockerin interaction.
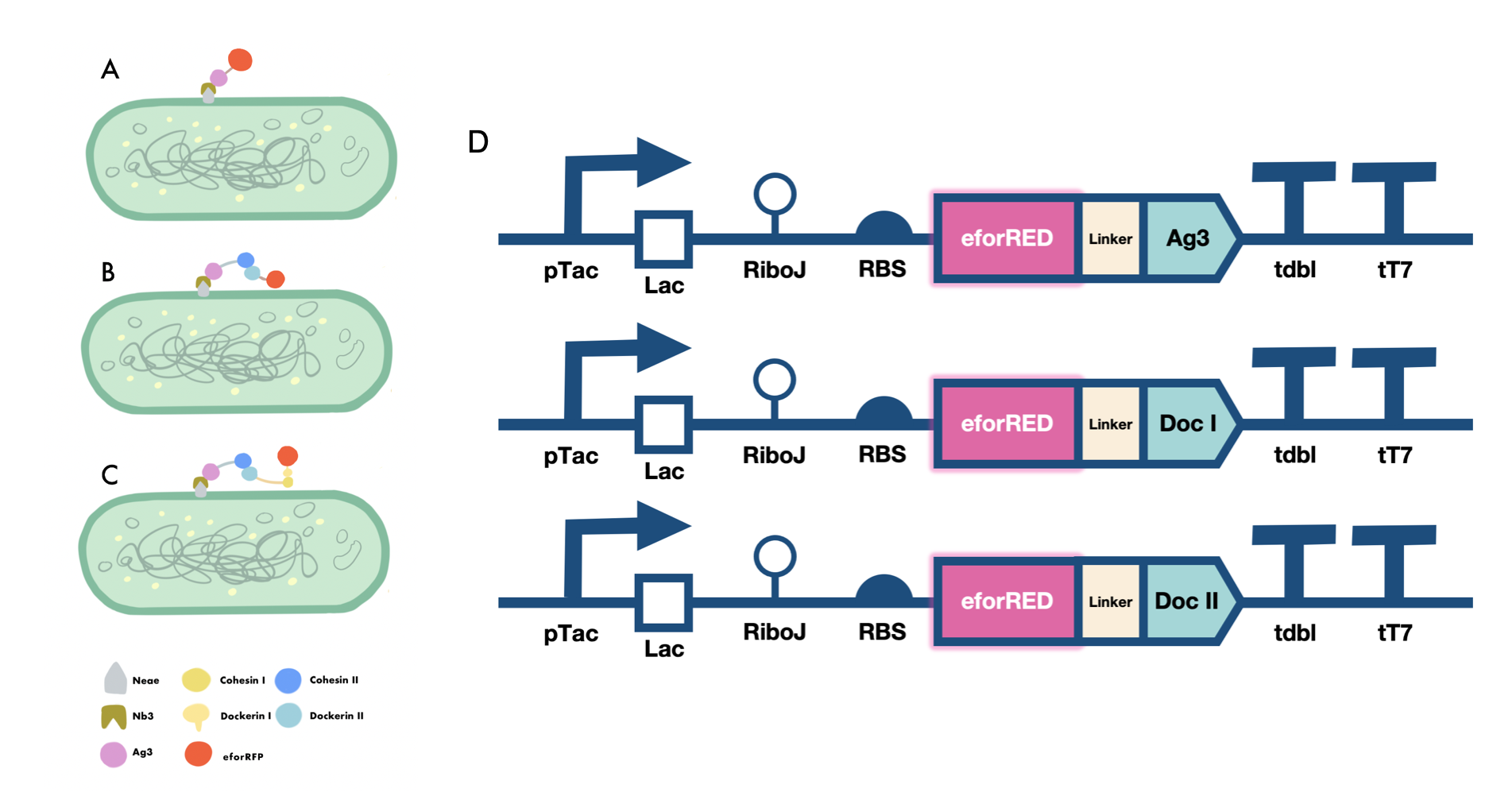
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
1. Brás, Joana L.A., et al. “Escherichia Coli Expression, Purification, Crystallization, and Structure Determination of Bacterial Cohesin–Dockerin Complexes.” Cellulases, 2012, pp. 395–415, 10.1016/b978-0-12-415931-0.00021-5.
2. Part: "Bba K592012 - Parts.Igem.Org". Parts.Igem.Org, 2022, https://parts.igem.org/Part:BBa_K592012.
| None |

