Part:BBa_K4274001
CmR29M2-ClSS_S533A
ClSS is a biobrick part encoding the gene for alpha-santalene synthase from Clausena lansium (GenBank: ADR71055.1). The enzyme catalyze the conversion of the common isoprenoid intermediate farnesyl pyrophosphate (FPP) into the alpha-santelene in a single step.The gene has been demonstrated to produce functional terpene product in both yeast (Wenlong Z., 2020) and E. coli (Jia Z. et al., 2022) .
CmR29, a DNA fragment comprising 87 nucleotides from the 5'end of the CmR gene, is a competent solubility enhancer in Synechocystis (Nico Betterle et al., 2018). CmR29M2 is a mutant CmR29 polypeptide for the expression of 3 different amino acids(Xun W. et al.,2021). Due to more than half of heterologous protein aggregated into insoluble inclusion bodies is an important limiting factor in the E. coli expression system, CmR29M2 fusion tag was linked to the N-terminus of ClSS, and the production of α-santalene reached 131% of the no-tag strain (Jia Z. et al., 2022).
Regarding to Jia Zhang's research, we constructed the fused CmR29M2-ClSS_S533A, and suggested that it might improve the production of santalene through enhancing the solubility of santalene synthase. In the future, other teams could use this part to improve the solubility of protein to improve the production of α-santalene when the gene coded for alpha-santalene synthase is heterologously expressed.
Usage and Biology
ClSS is a biobrick part encoding the gene for alpha-santalene synthase from Clausena lansium (GenBank: ADR71055.1). At the same time, with the mutation in ClSS's basic amino acid residue S533, the production of α-santalene could be boosted in E. coli (Jia Z. et al., 2022). And CmR29M2 is a fusion tag used to increase the solubility of α-santalene when ClSS is expressed in E. coli. Naturally, CmR29 is a solubility enhancer in Synechocystis (Nico Betterle et al., 2018), and CmR29M2 is a mutant CmR29 polypeptide for the expression of 3 different amino acids (Xun W. et al.,2021). A short stretch of CmR29M2 as a leader sequence can be added to the 5’end of ClSS_533A to increase the heterologous production of santalene (Nico Betterle et al., 2018).
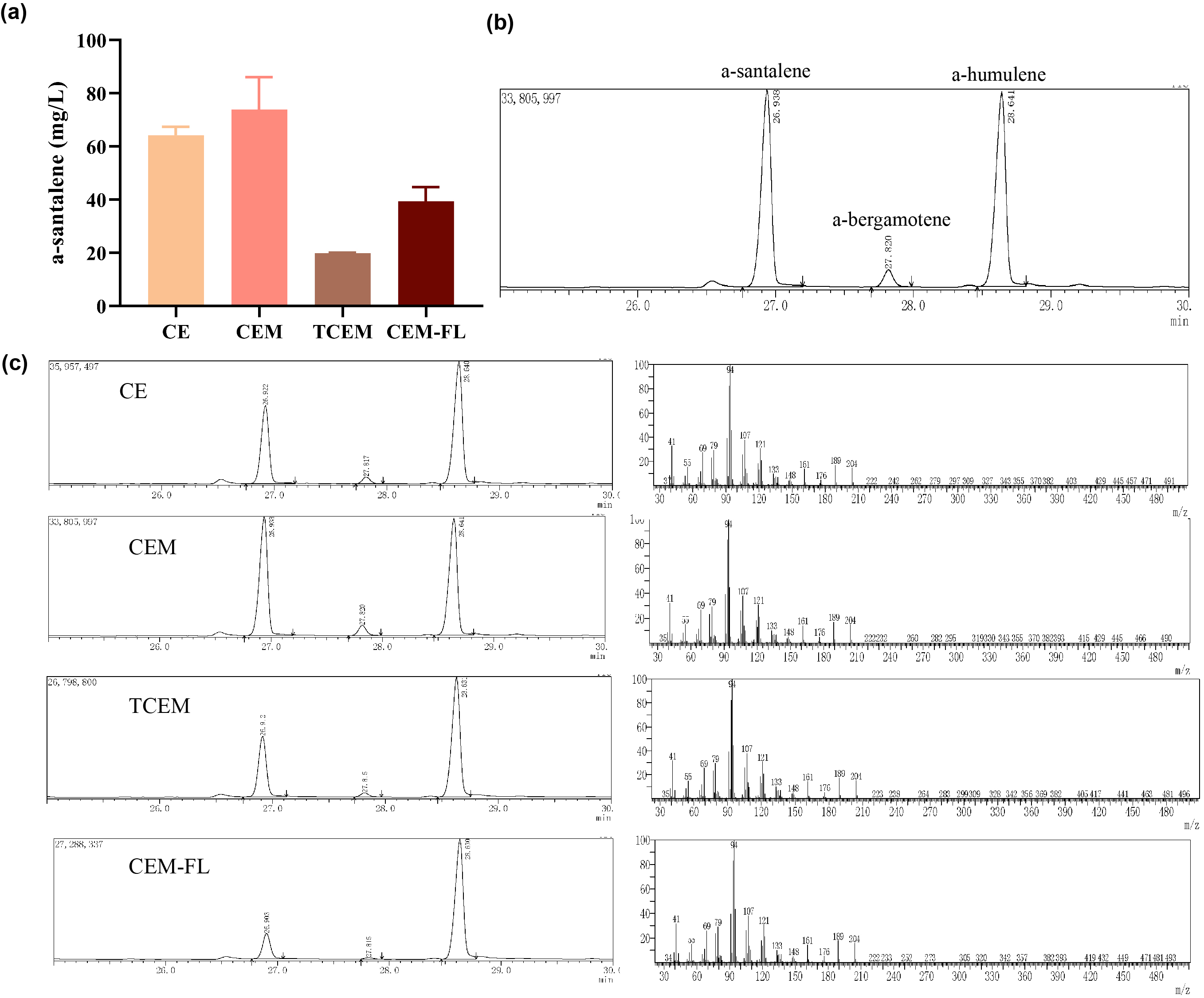
Characterization
With the help of the co-transformation of pMVA plasmid with various pW1 plasmids, including pW1_CE, pW1_CEM, pW1_TCEM and pW1_CEM_FL, different strains were successfully constructed and utilized for santalene production. The yield of santalene from strains CE, CEM, TCEM, CEM_FL could be used to characterize composite parts BBa_K4274030, BBa_K4274031, BBa_K4274032, BBa_K4274033. Eventually, strain CEM produces the maximal level of α-santalene, which elucidates that the mutation of 96th amino acid into tryptophan could increase the yield of α-santalene by about 20%. It substantiated the prominent performance of ERG20_F96W in enhancing the supply of FPP and α-santalene production in E. coli. However, hydrophilic tag of CmR29M2 resulted in a significantly decrease in santalene production.
Source
Clausena lansium; Synechocystis
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 270
- 1000COMPATIBLE WITH RFC[1000]
References
[1] Zha, W., An, T., Li, T., Zhu, J., Gao, K., Sun, Z., Xu, W., Lin, P., & Zi, J. Reconstruction of the biosynthetic pathway of santalols under control of the GAL regulatory system in yeast. ACS Synthetic Biology, 9(2), 449-456(2020). https://doi.org/10.1021/acssynbio.9b00479
[2] Jia Z., Xun W., Xinyi Z., et al. Sesquiterpene Synthase Engineering and Targeted Engineering of α-Santalene Overproduction in Escherichia coli. J. Agric. Food Chem. 70 (17), 5377-5385 (2022). https://doi.org/10.1021/acs.jafc.2c00754
[3] Betterle, N., & Melis, A. Heterologous leader sequences in fusion constructs enhance expression of geranyl diphosphate synthase and yield of β-Phellandrene production in cyanobacteria Synechocystis. ACS Synthetic Biology, 7(3), 912-921(2018). https://doi.org/10.1021/acssynbio.7b00431
[4] Wang, X., Chen, J., Zhang, J., Zhou, Y., Zhang, Y., Wang, F., & Li, X. Engineering escherichia coli for production of geraniol by systematic synthetic biology approaches and laboratory-evolved fusion tags. Metabolic Engineering, 66, 60-67 (2021). https://doi.org/10.1016/j.ymben.2021.04.008
| None |
