Part:BBa_K4184004
NpuN_NTEV
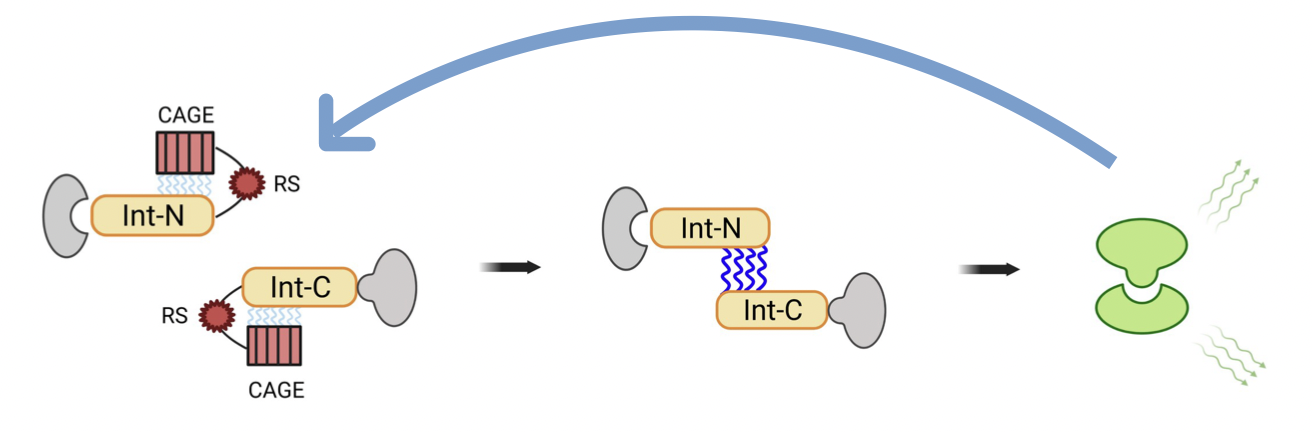
This part contains the N-terminal part of the TEV protase connected to the N-terminal part of an intein. A cage is attached to this intein part. This cage is a small part of a modified C-terminal intein. This prevents static interactions from occurring between the N-terminal part of an intein and the C-terminal part of an intein. Between the N-terminal part of the intein and the cage is the recognition site of the TEV protease. For the C-terminal part have a look at part: BBa_K4184003
This part contains Inteins. Inteins are proteins that possess autocatalytic activity. So they remove themselves from the host Protein. They do this with the so called protein trans splicing (PTS). The precursor proteins consists of three segments- an N-extein followed by the intein followed by a C-extein. During the protein splicing process, a significant change in the primary structure occurs, which removes the inteins and finally assembles the exteins.[1] The externs are then ligated back together by an amide bond[2].
In our project we used the inteins as an amplification component. If the protease is now present, it cuts off the cage at its recognition site and exposes the C-terminal part of the intein so that the N-terminal part, which has the same structure, can bind to it. Then both parts of the intein cut themselves out and the TEV protease is complete again and can activate further intein CAGE fragments. The fun part is that this System can amplify itself.
How to make inteins inducible: To make protein trans-splicing inducible one should ensure that the interaction does not occur without prior activation. This can be made possible by a new construction of the split-intein halves. Just previously, it was discovered that electrostatic interactions between an unstructured cationic region in the C-terminal fragment (residues 1-13) and an unstructured anionic region in the N-terminal fragment (residues 51-102) initiate complementation of the split intein pair [3]. Based on this discovery, the intein-constructs can be optimized by masking the regions important for interaction. For this purpose, the residues 51-102 of the N-terminal part and a linker were fused to the full-length C-terminal part of the intein and residues 1-13 of the C-terminal part as well as a linker were fused to full-length N-terminal part. This leads to a prevention of the interaction of both split-intein halves. To make the interaction inducible, a protease cleavage sequence is incorporated into the linker. Proteolytic removal of the cage sequences would trigger split intein association and hence PTS, as interaction of the split intein fusion partners is made possible again [4].
References: [1] Gramespacher JA, Burton AJ, Guerra LF, Muir TW. Proximity Induced Splicing Utilizing Caged Split Inteins. J Am Chem Soc. 2019 Sep 4;141(35):13708-13712. doi: 10.1021/jacs.9b05721. Epub 2019 Aug 21. PMID: 31418547; PMCID: PMC6903685. [2] Kang C, Shrestha KL, Kwon S, Park S, Kim J, Kwon Y. Intein-Mediated Protein Engineering for Cell-Based Biosensors. Biosensors (Basel). 2022 Apr 28;12(5):283. doi: 10.3390/bios12050283. PMID: 35624584; PMCID: PMC9138240. [3] (2) Shah, N. H.; Muir, T. W.; et al. J. Am. Chem. Soc. 2013, 135, 18673. [4] Gramespacher JA, Stevens AJ, Nguyen DP, Chin JW, Muir TW. Intein Zymogens: Conditional Assembly and Splicing of Split Inteins via Targeted Proteolysis. J Am Chem Soc. 2017 Jun 21;139(24):8074-8077. doi: 10.1021/jacs.7b02618. Epub 2017 Jun 7. PMID: 28562027; PMCID: PMC5533455.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |