Part:BBa_K4170055
SUMO-LbuCas13a coding device under rhaB promoter
This plasmid contains all the basic genetic parts required for the bacterial expression of codon-optimized LbuCas13a protein with the small ubiquitin-like modifier (SUMO) protein under the transcriptional control of rhamnose-inducible rhaB promoter regulated by rhaS and rhaR activators. Unlike the strong T7 transcriptional system which is characterized as “leaky” with hardly controlled expression intensity [2], the rhamnose system is often characterized by a slower response with very low basal transcriptional activity [3].
Biology and usage
CRISPR/Cas13a systems as RNA sensors
CRISPR-Cas systems are RNA-guided adaptive immune systems that protect prokaryotes from foreign genetic elements derived from evading viruses and phages (Shan et al., 2019). The Cas13a is ribonuclease with a double biological functionality: catalyzes the crRNA maturation and degrades the RNA-guided ssRNA (single-stranded RNA) interdependently using two separated catalytic sites (Rath et al., 2015). Generally, CRISPR/Cas system can be divided into two main classes, class I and II, according to the system comprising a single or multiple effectors (Liu et al., 2017). Among them, class II (e.g., Cas9, Cas12, and Cas13) possesses more widespread application, due to its simple components (a single effector protein and a programmable guide RNA, Wang et al., 2021). Cas13 can be further divided into four subtypes, Cas13a-d, exhibiting diverse primary sequences except the two highly conserved HEPN (higher eukaryotes and prokaryotes nucleotide-binding) domains, which are responsible for both cis- and trans-RNase activities (Florczuk et al., 2017). Structural studies revealed that Cas13a adopts a bilobed architecture including recognition (REC) and nuclease (NUC) lobes (Wang et al., 2021, Zhou et al., 2020). CRISPR-Cas12,13,14 exhibits nonspecific degradation of non target (trans cleavage) after specific recognition of nucleic acids, thus CRISPR/Cas biology promises rapid, accurate, and portable diagnostic tools, the next-generation diagnostics. Cas13a due to its propensity to cleave RNAs after binding a user-defined RNA target sequence, is used to detect single molecules of RNA species with high specificity (Liu et al., 2017).
Weaknesses in traditional RNA detection methods
This innovative molecular system has provided breakthrough opportunities for RNA detection for clinical diagnostics (Shan et al., 2019). Since RNA molecules are key mediators in all biological processes, served as significant biomarkers to the clinical verification and differential diagnosis of cancer. MiRNAs are a class of small non-coding RNAs that participate in numerous cellular processes such as cellular development, proliferation and apoptosis. Indeed, deviating miRNA expression is often related to the occurrence of diseases such as cancer. Therefore, the accurate detection of differences in miRNAs detection serve could serve as significant biomarkers for cancer verification and diagnosis. The RNA detection approaches can be characterized into 2 main categories, the direct detection and the indirect nucleic acid amplification (NAA)-based detection. Both approaches have advantages but also significant disadvantages which hinder their wide application in clinical practice. The direct classical methods including Nothern blotting, microarrays and FISH, despite their high fidelity, lack high sensitivity and are labor-intensive, time-consuming and require specialized personnel. On the other side, NAA-based detection approaches such as reverse transcription PCR (RT-PCR) and other isothermal amplification techniques show high sensitivity but introduce additional steps such as the conversion of RNA to DNA and auxiliary DNA template replication steps in order to achieve signal amplification. These additional steps often result in considerable sample loss due to incomplete RT step, amplification bias caused by error-prone sequence amplification, and a high risk of false-positive results due to possible contamination. Furthermore, the requirement of thermocycler for the reactions, limit its application as a point of care medical diagnostic device. These issues render the existing IVD tools for cancer diagnostics inadequate for clinical application in routine base.
SUMO solubility tag
SUMO protein has been previously fused to the N-terminus of several proteins leading to increased expression and solubility of the protein of interest (Liponska et al., 2018). But how does SUMO protein enhance protein solubility and proper folding? The answer lies in the structure of SUMO protein. Specifically, SUMO has an inner hydrophobic core and an external hydrophilic surface, thus exerting a detergent-like effect on proteins that cannot easily acquire their proper folding (Butt et al., 2005). Despite the advantages of SUMO addition, one drawback of this protein expression strategy is the necessary cleavage of the solubility tag. However, many SUMO proteases such as Ulp1, which are members of the cysteine protease superfamily can be used to cleave the SUMO tag without affecting the N-terminus of the desired protein (Panavas et al., 2009).
Basic parts of the device
The part sample which is flanked at the beginning and the end with prefix and suffix respectively, is composed of the following basic parts assembled together in series and downstream of the prefix:
- Bacterial terminator (for LacI CDS): putative bacterial transcription terminator
- Biobrick Prefix sequence: BioBrick (located at backbone)prefix for parts that do not start with "ATG"
- rhaR regulator: DNA-binding transcriptional activator for rhaSR, L-rhamnose binding
- rhaS regulator: DNA-binding transcriptional activator for rhaBAD and rhaT, L-rhamnose binding
- rhaB promoter: promoter of the E. coli rhaBAD operon, conferring tight induction with L-rhamnose and repression with D-glucose in the presence of RhaR and RhaS
- RBS based on Elowitz repressilator (BBa_B0034)
- 6XHis tags: 6xHis affinity tag
- Thrombin site: thrombin recognition and cleavage site
- bdSUMO CDS: small ubiquitin-like modifier (SUMO) protein
- LbuCas13a CDS: Cas13a protein derived from Leptotrichia Buccalis
- Biobrick Suffix sequence : universal suffix for all parts
- His operon terminator (downstream of Suffix) (located at backbone): putative transcription terminator from the E. coli
This genetic device is a part of the meta-CRISPR part collection developed by iGEM22_Thessaloniki_Meta.
Meta-CRISPR part collection
The meta-CRISPR part collection was developed in accordance with standardization, modularity and standard assembly rules aiming to enhance the CRISPR/crRNA applicability in synthetic biology projects. This collection allows the interchangeability of the genetic parts and the automation of construction following a standardized Golden Gate-based cloning strategy. Utilizing the meta-CRISPR collection, future iGEM teams can select the desired combination of DNA parts depending on the specific application of the CRISPR method (https://2022.igem.wiki/thessaloniki-meta/part-collection). Through the combination of different promoters and LbuCas13a coding sequences, one can select the desired LbuCas13a expression system, for example under the transcriptional control of the T7 or the pRha promoter. In addition, by combining the suitable genetic parts, researchers can select the purification method of the recombinant LbuCas13a protein either from inclusion bodies or from the soluble cytoplasmic fraction of the bacteria. Regarding the crRNA, utilizing the guidelines provided on the crPrep-crRNA preparation kit one can easily in silico design and produce the necessary crRNA sequence depending on the target miRNA utilizing the standardized cloning method and requiring only one additional primer. Last but not least, the LbuCas13a coding device can be assembled with the crRNA transcription system in a single plasmid enabling the simultaneous production of the LbuCas13a/crRNA complex in bacteria.

Design considerations
For the final assembly of the PCR amplified genetic elements into pSB1C3 plasmid we followed the Golden Gate-based ‘SevaBrick Assembly’ method and the SEVA 3.1 platform. As for all our final composite parts, the initial steps of our cloning strategy constitute the PCR amplification of different genetic elements, followed by the efficient assembly of the PCR amplified genetic products into pSB1C3 backbone. The cloning process is described in detail at the design section:
Cloning strategy and results
Step 1
- PCR amplification with Ep and Sp standard primers from Basic SevaBrick Assembly [seva 3.1] using the rhamnose Repos (BBa_K4170054) as a template. This PCR produces the rhaR-rhaS part ready for Golden Gate assembly.
- PCR amplification with Cas13a RBS FWD and P4 RVS primers using the SUMO-LbuCas13a Repos (BBa_K4170014) as a template. This PCR produces the Cas13a-RBS-P4 RVS part ready for Golden Gate assembly.
- PCR amplification with Ev and Pv standard primers from Basic SevaBrick Assembly [seva 3.1] using the Bba_J364007 part of the 2022 DNA distribution Kit. This PCR produced the pSB1C3 backbone linearized and ready for Golden Gate assembly.
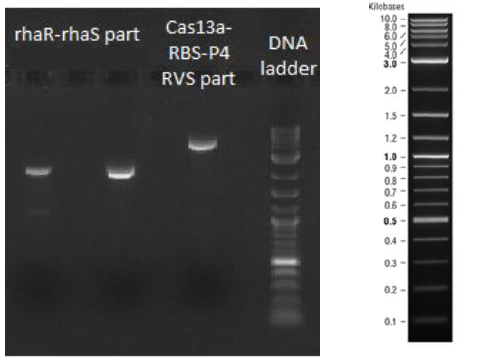
After the completion of the PCR amplifications, 1 % agarose gel electrophoresis was conducted. As shown in Figure 1 and 2 all the PCR amplifications succeeded. In figure 1 on the left side the rhaR-rhaS part (2119 bp) is depicted and on the right side the Cas13a-RBS-P4 RVS part (3903 bp).
Step 2
Golden Gate assembly of the PCR amplified rhaR-rhaS and Cas13a-RBS-R4 RVS parts with the linearized pSB1C3 vector for the efficient construction of the SUMO-LbuCas13a coding sequence under the transcriptional control of the rhamnose promoter.The products of the Golden Gate assembly underwent transformation into E.coli DH5a competent cells.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 289
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1318
Illegal BglII site found at 5543 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 1586
Illegal AgeI site found at 2406
Illegal AgeI site found at 3621 - 1000COMPATIBLE WITH RFC[1000]
Citations
1. Ke, Y., Huang, S., Ghalandari, B., Li, S., Warden, A., Dang, J., Kang, L., Zhang, Y., Wang, Y., Sun, Y., Wang, J., Cui, D., Zhi, X. and Ding, X., 2021. Hairpin‐Spacer crRNA‐Enhanced CRISPR/Cas13a System Promotes the Specificity of Single Nucleotide Polymorphism (SNP) Identification. Advanced Science, 8(6), p.2003611.
2. Du, F., Liu, Y., Xu, Y., Li, Z., Wang, Y., Zhang, Z. and Sun, X., 2021. Regulating the T7 RNA polymerase expression in E. coli BL21 (DE3) to provide more host options for recombinant protein production. Microbial Cell Factories, 20(1).
3. Wegerer, A., Sun, T. and Altenbuchner, J., 2008. Optimization of an E. coli L-rhamnose-inducible expression vector: test of various genetic module combinations. BMC Biotechnology, 8(1).
| None |