Part:BBa_K4170023
crRNA targeting the miR-17-3P (standard design)
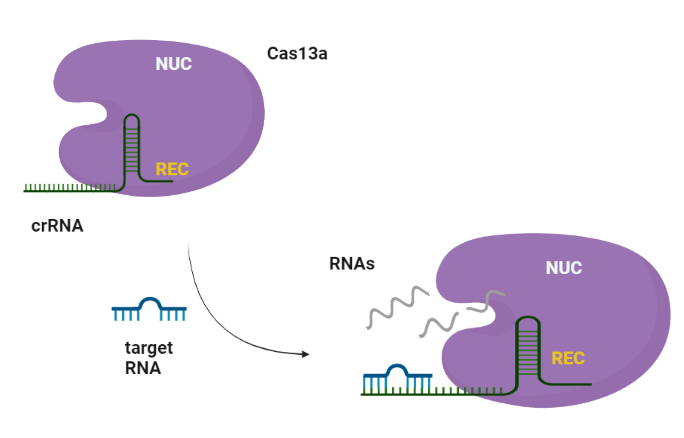
This part contains the sequences of the stem loop (repeat part) and the spacer (detection part) required for the formation of the CRISRPR/Cas13a complex and the hybridization with the mature miR-17-3P, respectively. The LbuCas13a protein adopts a bilobed structure which consists of a a-helical REC lobe and a NUC lobe. The crRNA stem loop sequence is anchored to the space between the NTD and Helical-1 domain forming extensive contacts between the crRNA and the LbuCas13a protein. The crRNA spacer region (guide) is complementary to the miR-17-3P.
Usage and biology
CRISPR/Cas13a system
CRISPR (Clustered regularly interspaced short palindromic repeats)-Cas (CRISPR-associated protein) systems are originally derived from prokaryotic adaptive immune system against invading nucleic acid components. Generally, CRISPR/Cas system can be divided into two main classes, class I and II, according to the system comprising a single or multiple effectors (Liu et al., 2017). Among them, class II (e.g., Cas9, Cas12, and Cas13) possesses more widespread application, due to its simple components (a single effector protein and a programmable guide RNA) (Wang et al., 2021). Cas13 can be further divided into four subtypes, Cas13a–d, exhibiting diverse primary sequences except the two highly conserved HEPN (higher eukaryotes and prokaryotes nucleotide-binding) domains, which are responsible for both cis- and trans-RNase activities. (Florczuk et al., 2017) Structural studies revealed that Cas13a adopts a bilobed architecture including recognition (REC) and nuclease (NUC) lobes (Wang et al., 2021, Zhou et al., 2020.
The Cas13a enzyme guided by the specific crRNA, possesses the target-dependent trans-cleavage of all nearby RNA molecules with high recognition ability and highly processive cleavage.
miRNAs and cancer
MicroRNAs (miRNAs) are a group of small non-coding RNAs of 17–25 nucleotides in length that are conserved across species. They were first discovered in Caenorhabditis elegans at the beginning of the 1990s. miRNAs are expressed in different tissues and cell types and are involved in regulating a range of developmental and physiological processes. Deregulated expression of these small RNAs have a significant impact on development of diseases including cancer. Many human miRNAs appear to control important processes that play vital roles in the onset, progression, and metastasis of cancer like:
- cell proliferation
- cell adhesion
- apoptosis
- angiogenesis
miR-17 and cancer association
MiR-17 is an oncogene that appertains to the MiR-17-92 cluster. This cluster is located in chromosome 13 and encodes for six individual miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a) (Concepcion et al., 2013). The miRNAs of this cluster are essential modulators of constitutive cellular processes like differentiation, metastases, apoptosis, proliferation. The carcinogenic role of miR-17-92 cluster in different cancers has been confirmed, while it seems to interact with the transcription factors E2Fs and c-Myc, which play a crucial role in cell-cycle regulation during tumorigenesis. As reported, miR-17 is significantly expressed in many types of cancer, including breast cancer, gastric cancer, prostate cancer, and NSCLC, and thus can be an efficient non-invasive biomarker for the screening of cancer patients.Recent literature has shown that in NSCLC, the mature strand mir-17-5p is associated mainly with LKB1/AMPK pathway and PTEN/PI3K/Akt pathway.
LKB1/AMPK pathway.
The Liver Kinase B1 (LKB1, also known as STK11) is an oncosuppressor gene, encoding a serine threonine kinase. LKB1 mutations are frequently associated with NSCLC. LKB1 positively regulates the AMP-activated protein kinase (AMPK) and at least 12 additional AMPK-related downstream kinases, involved in the control of cell growth , metabolism and in the regulation of cellular response to energy stress and establishment of cell polarity (Ciccarese et al., 2019). Deregulation of LKB1 signaling has been implicated in oncogenesis across many cancer types. Considering the scientific updates, overexpression of mir-17-5p seems to repress the levels of LKB1. Low levels of LKB1 influence the AMPK pathway and thus contribute to the deregulation of cell growth control (Borzi et al., 2021).
PTEN/PI3K/Akt pathway
Phosphatase and tensin homologue deleted on chromosome 10(PTEN) is a dual lipid and protein phosphatase. PTEN negatively regulates PI3K, leading to activation of the PI3K/Akt pathway. AKT activation stimulates cell cycle progression, survival, metabolism and migration through phosphorylation of many physiological substrates. Furthermore, PTEN influences the nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) protein levels, which is a key transcription factor for osteoclastogenesis (Carnero et al., 2008). Osteoclastogenesis is connected with bone metastasis in NSCLC. mir-17-5p appears to promote osteoclastogenesis, by interplaying with PTEN and activating the PI3K/Akt pathway (Wang et al., 2021).
Design methodology for crRNA targeting the miR-17-3P and in silico evaluation
The sequence consists of 2 main parts. The first part is called Repeat Part and it is a standard nucleotide sequence that binds to the Recognition Lobe of LbuCas13a and forms a stable complex with it. The second part is called spacer or detection part and it is responsible for the detection of the miR 17-3p. The Repeat part contains 31 nucleotides, and the spacer sequence is designed to contains 20 nucleotides that are complementary to the target miR sequence(Liu et al., 2017).
The first step of the in-silico evaluation is the evaluation of its thermodynamic properties. The software used for this task is ViennaRNA(Lorenz et al., 2011). The properties studied were : Minimum Free Energy, GC content of the sequence, Linearity of Detection Part, the Binding energy with the target miRNA and perfect matches.
The Minimum Free Energy is a measure of stability of the RNA and the negative it is the more stable will be. The Vienna Software is calculating the energy based on the secondary optimal structure. The value of -8 kcal/mol suggesting a relative stable structure. GC content is the ratio of the Guanine and Cytokine that are present in the sequence. The higher the ratio, the more stable the sequence will be. This phenomenon is based on the different chemical groups that are present in every nucleotide resulting in stronger hydrogen bonds in comparison with other nucleotides and the value 0.47 is suggesting a relatively stable structure. Linearity Of Detection Part (LODP) shows the secondary structure of the detection part of the crRNA sequence. Closer to 0 more linear will this part be. The linear structure of the detection part of crRNA is highly associated with the proper function of the LbuCas13a protein. The value of -1.9 kcal/mol is the result of unwanted hydrogen bonds in the spacer sequence, but since the value is low the miRNA-crRNA sequence interaction is energetically favorable. Binding energy is the energy term of the difference between the Gibbs free energy of the complex RNA and the free energy of crRNA and miRNA target. The binding energy is calculated using the secondary structure of the sequences. Since the value -46.2 kcal/mol of the binding energy is nearly 5 times more than the MEF of the crRNA sequence then the stability of the complex is the preferable energetical state. Perfect Matches is a measure of evaluation of the RNA complex that is formed by crRNA and miRNA target sequences. The secondary structure of the complex is calculated through RNAduplex, and perfect matches is the ratio of the nucleotides that are bound properly in the detection part of the crRNA sequences. The ratio of 1.0 is showing that the binding on the spacer sequence will be exactly as the suggested binding upon designing the sequence. The predicted secondary structure of the crRNA sequence and the complex formed between the crRNA and miRNA sequence are demonstrated on the next figure:
The next step for the in-silico evaluation is the molecular docking with the LbuCas13a protein. For this process, the utilized pdf file for LbuCas13a acquired from Protein Data Bank (ID 5XWY) and the pdf file for the crRNA 17-3p-standard sequence generated through the RNAComposer Sever. The docking algorithm was the HDOCK docking algorithm for the webserver(Zhang Di Yumeng Yan, 2017)The docking model selected was the best model that had the more negative value based on the scoring algorithm of HDOCK server(Wang et al., 2020)
The docking score is the energy score calculated through the scoring function of HDOCK algorithm(Zhang Di Yumeng Yan, 2017) The docking score is negatively related to the stability of the complex, meaning the more negative is the docking score more stable the complex receptor-ligand will be. The value of -439.53 kcal/mol shows that the complex formation is the energetical favorable state. The Ligand Root Mean Square Deviation is showing the difference between the positions of nucleotides of the ligand’s structure before and after docking. The ligand RMSD value of 50.31 Angstrom shows a major change in the conformation of the ligand which is acceptable since the ligand in this specific docking process is an RNA sequence of 51 nucleotides. The final step of the in-silico analysis is the molecular dynamics (MD) simulations. The MD simulations investigate the stability of the system and can measure the interaction energy between the protein and the crRNA sequence. Utilizing the same pdb files from the docking process, the MD simulations proceeded with the GROMACS software(Lindahl, Hess and van der Spoel, 2001) Using the pdb file of LbuCas13a and crRNA sequence and then after using the Amber force field, we utilized the pdb2gmx function of the GROMACS for the generation of topology for 2 the molecules. After the steps of the ionization and solvation, we equilibrated the system with respect to temperature and pressure. After these steps, the simulation began. The simulations of the systems were for a total time of 1 ns with time step of 1 ps. The first aspect of the data analysis is the Root Mean Square Deviation for the crRNA sequence. The Root Mean Square Deviation is representing the difference between two structures: a target structure and a reference. For the MD simulations for checking the system’s stability, we evaluate the difference between the stable final structure and the initial structure. The low values of RMSD shows the stability of the complex after its formation.
The other analysis that took place was the calculation of the Root Mean Square Fluctuation. The Root Mean Square Fluctuation is a calculation of individual residue flexibility, or how much a particular residue moves (fluctuates) during a simulation. It can be observed that the nucleotides in spacer part demonstrates higher values in comparison with the Repeat part meaning that the main interactions between protein and crRNA take place with the Repeat Part.
The next step of the analysis is the calculation of the Hydrogen Bonds formed with the crRNA sequence. The Hydrogen bonds are some of the most stable bonds that can be formed between 2 molecules. This is depicting their importance in biological molecules. The number of the hydrogen bonds seems to be stay the same after 450 ps meaning that the complex reach its stability before the end of the simulation.
Finally, in the next figure is demonstrated the binding energy of the LbuCas13a and the crRNA over time. The mean value of the binding energy calculated to be -2011.07 kJ/mol with standard deviation +- 155.63 kJ/mol. The main energy that contributed was the Coulomb interactions meaning that the nature of the interactions between the two molecules are mainly electrostatic.
General methodology for the in silico design and experimental production of any desired crRNA.
Seeking to simplify the in silico design and experimental process for the production of a functional miRNA-targeted crRNA, we introduce a methodology for the crRNA preparation. Detailed information regarding this methodology is provided on the crPrep crRNA preparation kit section on the contribution page of Thessaloniki_Meta. The provided guidelines cover the entire crRNA developmental process from in silico design to in vitro transcription and allow future iGEM teams to efficiently easily produce the desired crRNA even if they do not have significant expertise in designing genetic sequences for cloning applications. In addition, the desired crRNA sequence can be inserted in any available pSB#X# biobrick plasmid just by choosing the desire pSB#X# as a template for the PCRs. Further inforamtion regarding the basic principle and design considerations can be found at the follwoing sections.
Basic principle and design considerations of the method
The basic principle of the method is the PCR amplification of any biobrick pSB#X# plasmid utilizing two sets of standardized primers. These primers have additional overhang sequences which correspond to the sequences of the crRNA spacer and loop respectively flanked by the BsaI recognition sequences on their 5’ edge. After PCR amplification of the pSB1C3 plasmid, the first primer set incorporates the loop region of the crRNA in the amplified DNA product, while the second pair incorporates the desired crRNA spacer region in the corresponding PCR product. In addition, each primer set integrates suitable BsaI recognition sites on the edges of the amplified PCR products generating sticky ends which allow for the efficient assembly of the two amplified DNA sequences utilizing Golden Gate assembly.
- The loop FWD standard primer hybridizes with the pSB1C3 DNA region which corresponds to the sequence presented between the origin of replication (ori) and lambda t0 terminator of the pSB1C3 backbone.
- The loop RVS standard primer hybridizes with the pSB1C3 DNA region which corresponds to the biobrick prefix sequence of the pSB1C3 plasmid. This primer has an additional overhang sequence which corresponds to the T7 promoter along with the crRNA stem loop region.
- The spacer FWD interchangeable primer hybridizes with the biobrick suffix sequence of the pSB1C3. This primer has an additional overhang which corresponds to the desired crRNA spacer region. This region needs to be modified depending on the desired target miRNA of the CRISPR/Cas13a system.
- The spacer RVS standard primer hybridizes with the 3’ edge of the pSB1C3 origin of replication.
As described above, 3 of the 4 required primers show conserved and identical nucleotide sequences regardless of the target miRNA. Thus, the only primer that needs to be modified according to the target miRNA is the spacer FWD interchangeable primer.

In silico design of the spacer FWD interchangeable primer
As described above, the only primer which needs modifications depending on the desired target miRNA sequence is the spacer FWD interchangeable primer. The sequence noted with red bold color should be replaced by the DNA sequence which is complement to the miRNA target, however with 3’ to 5’ direction i.e upside down .

Guidelines on cloning strategy.
To efficiently construct the final plasmid with the desire crRNA insert under the transcriptional control of the T7 promoter, the following steps should be followed:
Step 1.PCR amplification
- PCR amplification with loop RVS standard and loop FWD standard primers using the PSB1C3 plasmid as a template. These primers amplify a specific region of the pSB1C3 plasmid sequence (lambda to terminator, CmR-chloramphenicol acetyltransferase, prefix) and introduce the loop sequence of the crRNA to the amplified PCR product. This PCR produces the loop part ready for Golden Gate assembly. The loop part is ready to use for any crRNA constructed with this method since the stem-loop sequence is universal for all crRNAs.
- PCR amplification with spacer FWD interchangeable and spacer RVS standard primers using the PSB1C3 plasmid as a template. These primers amplify a specific region of the pSB1C3 plasmid sequence (ori-origin of replication, his operon terminator, suffix) and introduce the spacer sequence of the crRNA to the amplified product. This PCR produces the spacer part ready for Golden Gate assembly.
Step 2. Golden Gate assembly
- Golden Gate-based SevaBrick assembly () of the PCR amplified loop part and spacer part for the efficient construction of the crRNA coding sequence under the transcriptional control of the T7 promoter in the pSB1C3 plasmid.
Step 3. Bacterial transformation and plasmid DNA extraction
- The assembly reaction is transformed into chemically competent E.coli DH5a cells using heat shock transformation.
- Isolation of the crRNA-pSB1c3 plasmid using any commercial plasmid DNA isolation and purification kit.
Guidelines for in vitro Transcription
Step 1. Linearization and purification
- Plasmid linearization utilizing the SapI restriction enzyme. To produce the crRNA with the defined length, the plasmid DNA should be linearized downstream of the inserted crRNA sequence.
- Linearized DNA template band purification utilizing any commercial DNA Gel Extraction Kit
- DNA template purification by phenol/chloroform extraction
Step 2. In vitro transcription
- The purified linear DNA template serves as a template for in vitro transcription utilizing T7 RNA polymerase. Any commercially in vitro transcription kit can be used to synthesize sufficient amounts of the desired crRNA.
- crRNA purification with any available RNA purification kit which allows for the efficient purification of small length RNAs.
Results of the cloning procedure followed to construct the crRNA in vitro transcription device (BBa_K4170019)
The plasmid with the crRNA targeting hte miR-17-3P (standard design) under the T7 promoter for the in vitro transrciption was constructed with direct PCR amplification of PSB1C3 plasmid utilizing 2 pairs of primers followed by a Golden Gate-based ‘SevaBrick Assembly’ method (Damala et al., 2020).
'SevaBrick assembly' method
The Golden Gate-based ‘SevaBrick Assembly’ method was introduced by Stamatios G. Damalas and colleagues (Damalas et al., 2020). This method consists of standardized primers and protocols and facilitates the straightforward one-step assembly of multiple genetic elements into the SEVA 3.1 or the pSB#X# (# is determined by the identity of the replication origin and the letter X is determined by the antibiotic resistance marker) backbones in a fast and reliable process. The SevaBrick Assembly is a method where all the parts and backbones to be assembled are PCR amplified from any SEVA 3.1 or BioBrick vector, using a core set of standard long primers. All SevaBrick primers anneal on standard sequences of the SEVA 3.1 or BioBrick vectors, introducing BsaI recognition sites for directional multipart assembly via Golden Gate (Figure 2).
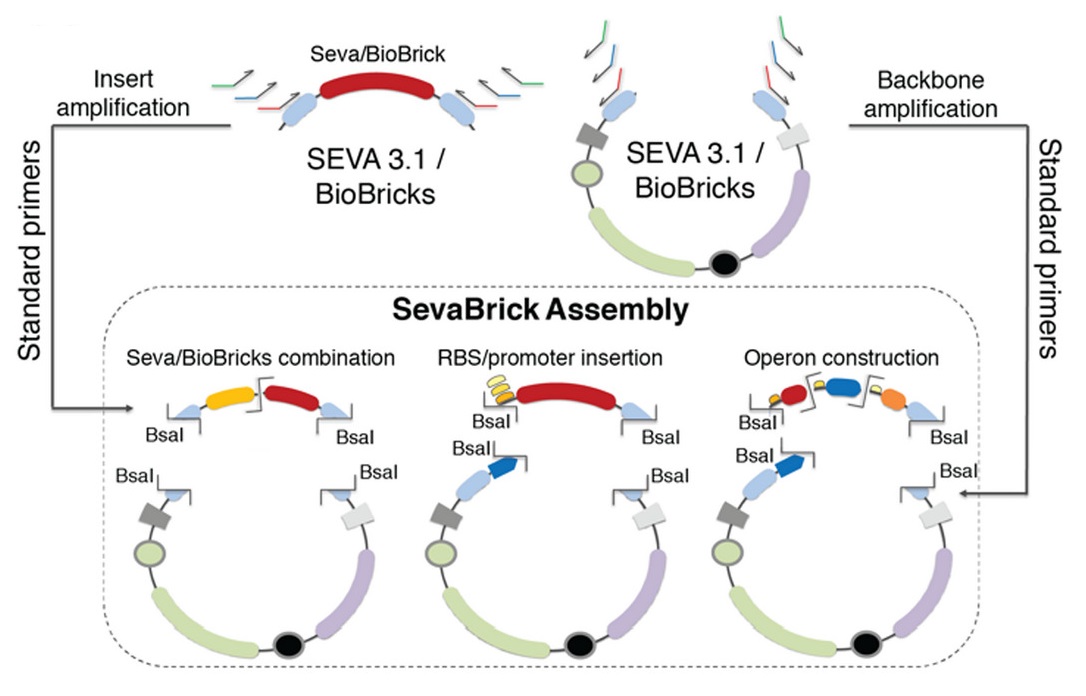
The first pair consists of a primer (loop RVS standard) binding to the Biobrick prefix sequence containing an overhang with the stem-loop sequence of the crRNA and a primer (loop FWD standard) binding to the backbone of the plasmid. The second pair of primers consists of a primer (3ps spacer FWD interchangeable) binding to the Biobrick suffix sequence containing an overhang with the spacer sequence of the crRNA, and a primer (spacer RVS standard) binding to the backbone of the plasmid. This strategy enables the handy and fast alternation of the overhang sequence of the interchangeable primer for the construction of any crRNA flanked in PSB1C3 plasmid keeping the other primers as customary components. The plasmid contains all the basic genetic parts required for the in vitro transcription of the crRNA-3ps regulated by T7 promoter.
Step 1
- PCR amplification with loop RVS standard and loop FWD standard primers using the PSB1C3 plasmid as a template. These primers produce the loop sequence of the crRNA incorporated with the CmR sequence (confers resistance to chloramphenicol) of the PSB1C3 plasmid. This PCR produces the loop part ready for Golden Gate assembly. The loop part is ready to use for any crRNA constructed with this method as the stem-loop sequence is universal for all crRNAs.
- PCR amplification with 3ps spacer FWD interchangeable and spacer RVS standard primers using the PSB1C3 plasmid as a template. This PCR produces the 3ps-spacer part ready for Golden Gate assembly.

According to the results of the agarose gel electrophoresis, all PCR amplification were succesful.
Step 2
Golden Gate assembly of the PCR amplified loop part and 3ps-spacer part for the efficient construction of the crRNA-3ps coding sequence under the transcriptional control of the T7 promoter. The Golden Gate assembly products underwent transformation into E.coli DH5a competent cells and then colony PCR was performed, using the primers VR and VF2. Picking sample from different colonies and then evaluating the results on a 1 % agarose gel electrophoresis we concluded that the Golden Gate assembly was successful. As depicted on the following figure from all colonies the desired inserts were successfully amplified. For the colony PCR procedure, from the agar plate half amount of each colony was picked and diluted on 10 μl of dH20. The other half amount was picked for liquid overnight culture
In vitro transcription of the crRNA-17-3P under the control of T7 promoter (BBa_K4170019)
The first step for the in vitro transcription of the crRNA-17-3P is the plasmid linearization through digestion with the restriction enzyme SapI. The SapI recognition site is located downstream of the crRNA sequence.
The linearized DNA template bands were purified by Nuclospin Gel and PCR Clean-up kit (Macherey-Nagel, Duren, Germany) and a second purification step with phenol/chloroform was performed.
The next step was the template-directed synthesis of the crRNA through in vitro transcription using T7 Polymerase (HiScribe T7 High Yield RNA Synthesis Kit, NEB) and the purification of the products, using Monarch RNA Cleanup Kit (NEB). In the following figure the crRNA transcripts are depicted on a 3% agarose gel electrophoresis after in vitro transcription. Additional non-selective RNA products are depicted at higher molecular weight.
The crRNA transcripts are further purified from other non-selective RNA products through a second clean-up procedure with the PCR Clean-up kit (Macherey-Nagel, Duren, Germany). The results of the additional clean up step are depicted on the following figure.
Kinetics of Cas13a/crRNA reaction depending on different reporter concentrations
Brief introduction
iGEM22_Thessaloniki_Meta developed DIAS, a detection system for the early diagnosis of lung cancer based on miRNA biomarkers. This system is based on Cas13a enzyme which is guided by a specific crRNA for the recognition of the target miR-17-3P. The crRNA hybridizes with the target miRNA and triggers the Cas13a enzyme trans cleavage activity against any nearby RNA molecules. In the reaction, a specific concentration of an FQ5U RNA reporter is added. After activation caused by the target miRNA, the Cas13a digests the RNA reporter causing the releasing of fluorescent signals.
To develop a detection system with increased sensitivity and broad clinical applicability a lot of factors affecting the CRISPR/Cas13a enzyme reaction need to be investigated.The system's detection method is based on a detection standard curve; a bioanalytical method which represents a linear relationship between concentration of an analyte (independent variable-miRNA added) and response (dependent variable-fluorescence intensity). Finally, this relationship is used to predict the unknown concentration of the analyte in a complex matrix such as the blood or serum. To achieve the detection system's maximum effectiveness we had to investigate factors affecting the enzyme reaction such as the appropriate reporter concentration and the specific time point since reaction initiation that the fluorescence signal should be obtained.
Brief experimental procedure
For the investigation of factors that affect the kinetics of the reaction such as the optimal reporter concentration we designed a simplified detection protocol. The basic steps of the protocol are the preparation of the CC reaction mixture along with the different RS-P and RS-N reaction mixtures as described in detail below.
RS reaction mixtures preparation
The RS-P positive reaction mixtures contained a predefined concentration of target miRNA-17-3P (1nM) in 1X reaction buffer. However, different concentrations of Reporter RNA (1μM, 0.75 μΜ, 0.5μΜ, 0.25μΜ, 01μM) were added in each reaction mixture to investigate the optimal reporter concentration for the enzyme reaction. In addition, the RS-N negative reaction mixtures contained the same RNA reporter concentration as for the RS positive reaction mixtures without the addition of the target miRNA. Each RS reaction mixture has 30μl volume.
CC reaction mixture preparation
The CC final reaction mixture contains 20nM LbuCas13a, 20nM crRNA in 1X reaction buffer.The CC reaction mixture has 20μl volume.
Microplate preparation for fluorescence experiments
After CC reaction mixture incubation at 37 °C and RS reaction mixtures preparation, a specific volume of the CC mixture (20μl) was added to each RS mixture generating the final detection master mixtures which were gently flicked. Afterwards, 50μl of the final detection master mixtures were added to each corresponding well of the microplate. The microplate was incubated for 60 min in a fluorescence plate reader with fluorescence measurements (FAM channel, 494 nm, 518 nm) taken every 1 mi
From the real time kinetic measurement of Cas13a reactions incubated with different reporter concentrations and a predefined miRNA concentration (1nM), we observed a conservative pattern on how the fluorescence intensity changed over time. During the first minutes of the reaction the emitted fluorescence from the RNA reporter increases linearly until the reaction reaches the plateau phase when the fluorescence intensity starts to decrease. On the contrary, the negative control samples exhibit constant background fluorescence at all time points.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Citations
1.Borzi, C., Ganzinelli, M., Caiola, E., Colombo, M., Centonze, G., Boeri, M., Signorelli, D., Caleca, L., Rulli, E., Busico, A., Capone, I., Pastorino, U., Marabese, M., Milione, M., Broggini, M., Garassino, M., Sozzi, G. and Moro, M., 2021. LKB1 Down-Modulation by miR-17 Identifies Patients With NSCLC Having Worse Prognosis Eligible for Energy-Stress–Based Treatments. Journal of Thoracic Oncology, 16(8), pp.1298-1311.
2.Carnero, A., Blanco-Aparicio, C., Renner, O., Link, W. and Leal, J., 2008. The PTEN/PI3K/AKT Signalling Pathway in Cancer, Therapeutic Implications. Current Cancer Drug Targets, 8(3), pp.187-198.
3.Ciccarese, F., Zulato, E. and Indraccolo, S., 2019. LKB1/AMPK Pathway and Drug Response in Cancer: A Therapeutic Perspective. Oxidative Medicine and Cellular Longevity, 2019, pp.1-16.
4.Concepcion, C., Bonetti, C. and Ventura, A., 2012. The MicroRNA-17-92 Family of MicroRNA Clusters in Development and Disease. The Cancer Journal, 18(3), pp.262-267.
5. Damalas, S., Batianis, C., Martin-Pascual, M., Lorenzo, V. and Martins dos Santos, V., (2020) "SEVA 3.1: enabling interoperability of DNA assembly among the SEVA, BioBricks and Type IIS restriction enzyme standards." Microbial Biotechnology, 13(6), pp.1793-1806.
6.Lindahl, E., Hess, B. and van der Spoel, D. (2001) ‘GROMACS 3.0: A package for molecular simulation and trajectory analysis’, Journal of Molecular Modeling, 7(8), pp. 306–317. Available at: https://doi.org/10.1007/S008940100045.
7.Liu, L. et al. (2017) ‘The Molecular Architecture for RNA-Guided RNA Cleavage by Cas13a’, Cell, 170(4), pp. 714-726.e10. Available at: https://doi.org/10.1016/j.cell.2017.06.050.
8.Lorenz, R. et al. (2011) ‘ViennaRNA Package 2.0’, Algorithms for Molecular Biology, 6(1), pp. 1–14. Available at: https://doi.org/10.1186/1748-7188-6-26.
9.Wang, L. et al. (2020) ‘Rapid design and development of CRISPR-Cas13a targeting SARS-CoV-2 spike protein’, Theranostics, 11(2), pp. 649–664. Available at: https://doi.org/10.7150/thno.51479.
10.Wang, M., Zhao, M., Guo, Q., Lou, J. and Wang, L., 2021. Non-small cell lung cancer cell–derived exosomal miR-17-5p promotes osteoclast differentiation by targeting PTEN. Experimental Cell Research, 408(1), p.112834.
11.Zhang Di Yumeng Yan (2017) ‘HDOCK : a web server for protein-protein and protein -DNA/RNA docking based on a hybrid strategy’.
| None |