Part:BBa_K3900000
Ribose Binding Protein NNK Sequence for Darwin-Assembly
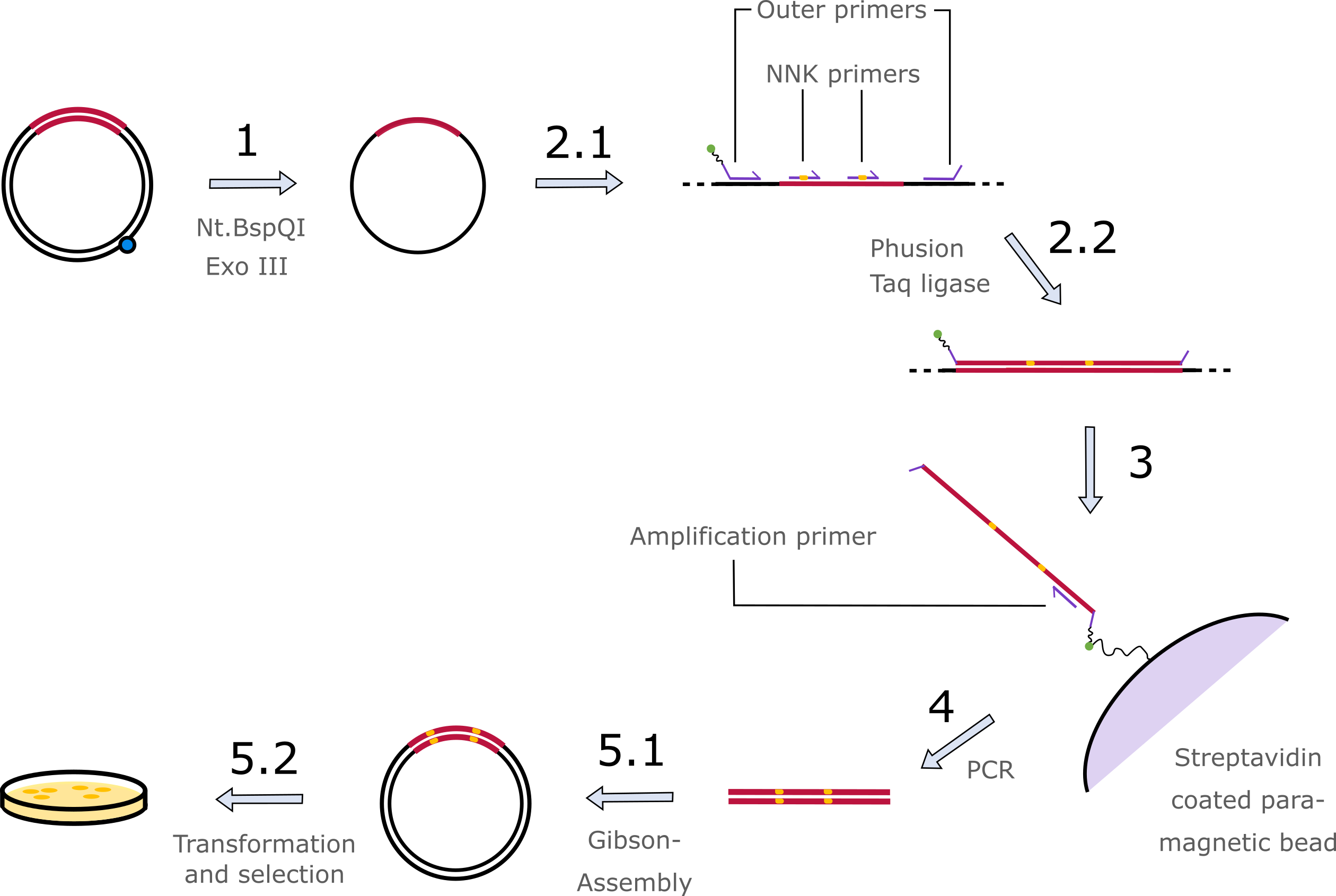
This part is the library version of the Ribose Binding Protein (RBP, part BBa_K3900001). The library was created using the Darwin Assembly. [1]
The Darwin Assembly allows the mutation of single amino acids by introduction of randomized bases. We took the RBP sequence, which we also used in the computational protein design, as basis for the library creation.
Therefore, we cloned the gene into the plasmid pJOE5751.1 by Gibson assembly and created ssPlasmids by nicking one strand with Nt.BspQI and digesting it with Exonuclease III. The RBP-sequence is flanked by a 5´-biotin-marked and a 3´-nuclease and polymerase-protected oligonucleotid that bind the RBP gene on both ends in the same orientation. NNK-oligonucleotids bind at the positions of the amino-acids of interest. Using a Phusion polymerase and Taq ligase, RBPs with randomly exchanged target triplets are created. Those can be purified using paramagnetic streptavidin beads that bind the biotin at the 5´-end of the fragments [2].
While still being bound to the streptavidin beads the fragments are amplified using PCR and then inserted into the plasmid backbone pJOE5751.1 via Gibson Assembly. This plasmid is then transformed into cells that contain the signaling cascade plasmid with an antibiotic-resistance instead of GFP. In the last step positive mutated clones are selected on agar that contains an antibiotic and one of the chemicals we want to test. Thereby, only mutants with a functional receptor, which is able to bind the given chemical, survive (Figure 1).
[1] Cosenz, C. & Pinheiro, V. B. Darwin Assembly: fast, efficient, multi-site bespoke mutagenesis. Nucleic Acids Research 48, 51ff (2018).
[2]Lambert, K. N. & Williamson, V. M. cDNA Library Construction Using Streptavidin-Paramagnetic Beads and PCR. The Nucleic Acid Protocols Handbook 41, 289-294 (2000).
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 385
Illegal PstI site found at 601 - 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 385
Illegal PstI site found at 601 - 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 385
Illegal PstI site found at 601 - 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 385
Illegal PstI site found at 601
Illegal NgoMIV site found at 480 - 1000COMPATIBLE WITH RFC[1000]
| None |