Part:BBa_K3716012
6xHis-Beta-glucuronidase (E.coli, gusA)
6xHis-Beta-glucuronidase (E.coli, gusA)
Design
This is a basic part. One can refer to the following composite parts that contains this basic part.
T7-LacO-gusA (E. coli), BBa_K3716022.
Introduction
This year team iBowu-China used this part to express the enzyme called β-glucuronidase in E. coli strain BL21(DE3) and similar variants of the strain. The designed purpose of this enzyme is to convert glycyrrhizic acid into glycyrrhetinic acid, which is the effective active ingredient of licorice.
Protocol
- Transform the plasmids into E. coli BL21(DE3)
- Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 4ml LB medium with kanamycin. Add 1mM IPTG to all experimental groups as needed. Incubate at 37℃ in a shaker overnight.
- Fluorescence. Acquire 100 ul bacteria culture and centrifuge at 10000g for 1 min. Aspirate the supernatant and resuspend with 100 ul ddH2O to get rid of the fluorescence background from LB medium. Add 100 µl sample solution into a sterile 96-well plate. Measure fluorescence with a microplate reader and then also measure OD600 for normalization.
- SDS-PAGE.
- Take 1ml of bacterial solution, centrifuge at 12000g for 1min at 4℃, discard the supernatant, add 100ul of pre-cooled RIPA lysate (high), vortex to mix, settle for 5min, centrifuge at 4℃, 12000g for 10min, and take the supernatant which contains the protein extract. After protein quantification by BCA method using commercial test box, adjust the concentration of the protein solution to 1mg/ml. Take 4 parts of the protein solution and 1 part of 5X protein loading buffer and mix it in a boiling water bath for 5 minutes, centrifuge at 12000g at 4°C for 10 minutes, take the supernatant, and store on ice. Use precast gel purchased from commercial company (Transgen, Precast Tris-Glycine Gel) for protein electrophoresis.
- Add marker in the first lane on the left at 5μl, and add sample on all other lanes with 10ul sample on each lane. Use 100V for electrophoresis. After the electrophoresis, take out the gel, put it in the staining box, add Coomassie Brilliant Blue dye solution to about 3mm below the gel surface, shake on a horizontal shaker, dye for 30min at room temperature, discard the dye solution, wash off the floating color with distilled water, and add decolorization liquid, decolorize on a horizontal shaker until the result is in good condition for taking pictures.
- Enzyme Activity
- Take 100 ul of bacterial solution, centrifuge at 10000g for 1min at room temperature and discard the supernatant. Resuspend with 100ul ddH2O, and add into this solution 100ul of standard test solution I (containing Phenolphthalein-β-D-glucuronide, and the test box was purchased from Nanjing Jiancheng Biotech company). Incubate the mixture at 37C for 1 hour.
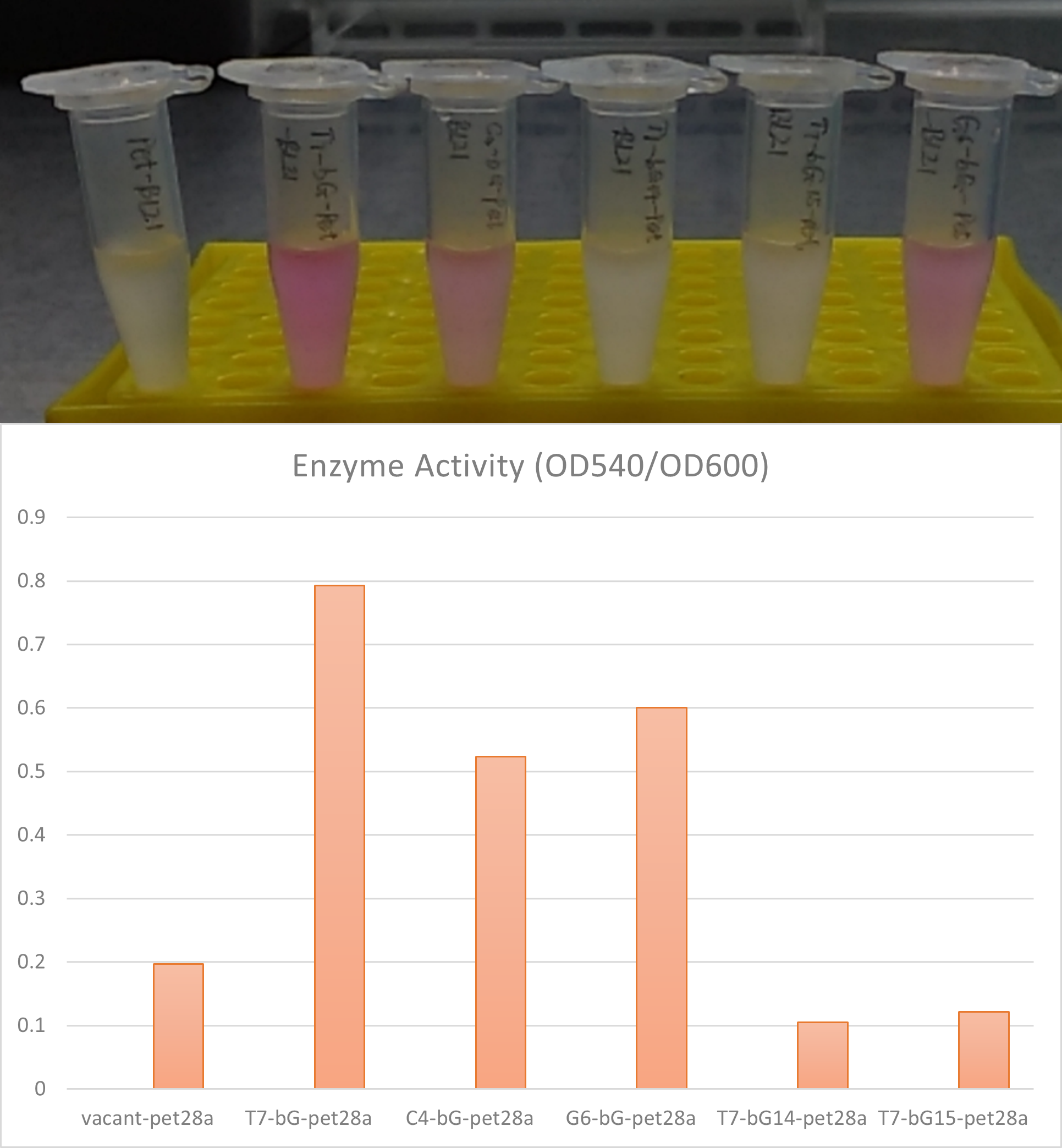
- Observe the solution. pink or purple color indicates positive enzyme activity. Quantitatively the activity can be measured by OD540 reading on a microplate reader, divided by its OD600 reading for normalization of concentration.
Results
1. Confirmation of the Gene Circuit with sfGFP
First we needed to experimentally confirm that our gene circuit would work as we had expected. We used sfGFP (Part:K515105) gene to substitute the β-glucuronidase gene in the above plasmid construction. The reconstructed plasmid was then cloned in DH5a and then extracted to be transformed into BL21(DE3). With the induction Iptg added, we can clearly measure strong signal of green fluorescence light. The intensity is about 100 fold stronger than the background, which is obtained by measuring the same bacteria culture but without Iptg induction. This result confirm that with Iptg induction, our gene circuit works normally and can be used for expression of bG enzyme.
2. Successful Expression of the Protein with SDS-PAGE
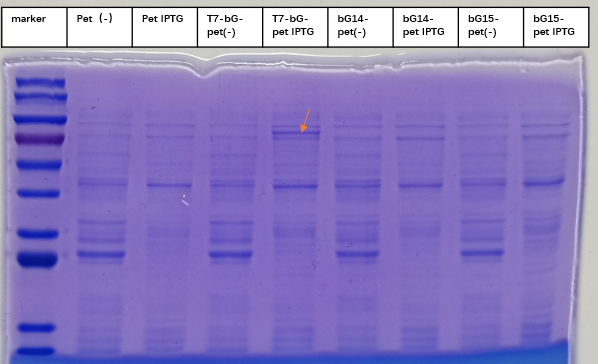
To express the enzyme β-glucuronidase, the plasmid was clone in DH5a and then extracted to transform BL21(DE3). Iptg was added at 1mM at OD600=0. The medium was incubated at 37 degree Celsius with shaking overnight, and then taken to SDS-PAGE experiment to check for the expression of the enzyme. This enzyme, which we named T7-bG-pet, was expected to have a size about 67 kDa, and the SDS-PAGE result showed the successful expression of the protein at the expected size band (marked by a red arrow on the figure). We included a control group of vacant-pet28a, of which the only difference is there the β-glucuronidase sequence is removed. Control groups where there is no Iptg induction for the same plasmid and control groups of vacant-pet28a (labeled Pet) showed no intrinsic protein exists in the expected bands.
3. Measurement of the Function of the Enzyme
Measurement of enzyme activities can be easily achieved by using β-glucuronidase to hydrolyze specific substance in a buffer and measure the hydrolysis product. Here we used Phenolphthalein-β-D-glucuronide as the substrate and after hydrolysis reactions with β-glucuronidase, free phenolphthalein will be released into the buffer. The system would show a pink to purple color after adding NaOH solution at a prepared standard concentration. The activity can also be quantitatively measured by measuring the light emission at 540 nm, which is the most intensive emission of colorant.
In the measurement, we added a control group labeled vacant-pet, where the plasmid and the gene circuit is the same, but the β-glucuronidase is removed. The control group went through exactly the same process of incubation and induction with Iptg to provide a background reading.
We also compare between the β-glucuronidase sequence from E. coli source (T7-bG-pet), Aspergillus oryzae source (bG14-pet), the Thermotoga maritima source (bG15-pet) and also a control group (vacant-pet). We found the enzyme encoded by the E. coli source (T7-bG-pet) has a significantly higher enzyme activity compared to others; and the enzyme activity from Aspergillus oryzae source and Thermotoga maritima source is not stronger than the background. Previous research works reported positive enzyme activities of the β-glucuronidase enzyme from these two sources, but here in our experiment conditions these two enzymes do not display discernible performance.
Summary The gene circuit constructed in pet28a is proper for the expression of β-glucuronidase enzyme. The enzyme from E. coli source has shown relatively high activity while the activities of the enzymes from the two other sources (Aspergillus oryzae and Thermotoga maritima) are low. We believe the expression conditions for the latter two enzymes would have to be further explored to achieve good catalytic hydrolysis performance.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |