Part:BBa_K3389003
RAD27 Flappase expression construct with 10x His tag
This part codes for an expression system for the RAD27 flap endonuclease 1 ("flappase") from Saccharomyces cerevisiae, including a 10x His tag for purification purposes. RAD27, also known as FEN-1, is a structure-specific flap endonuclease that will cleave overlapping oligonucleotides at the site of a single target nucleotide in the context of a known sequence. One use for this is to distinguish between single nucleotide polymorphisms. The expression system has a T7 promoter, a strong RBS, and a 10x His tag.
Characterization
In order to test the expression of the Flappase protein, we set out to test various concentrations of IPTG to induce production and determine the optimal length of incubation prior to harvesting the cells. We transformed the RAD27 Flappase part (BBa_K3389003) into BL21 DE3 competent cells using our transformation protocol. We confirmed a successful transformation via colony PCR and restriction digest (see Team SUNY_Oneonta 2020 wiki).
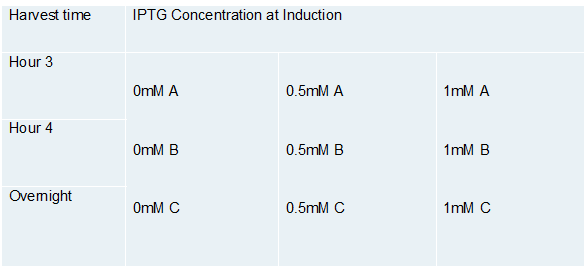
Figure F-02 summarizes the small-scale expression experiments conducted to determine optimal conditions. In these experiments, each test tube contained 3mL of the transformed Flappase construct as a liquid culture with 25 ug/mL chloramphenicol. Cultures were grown until they obtained OD600=0.600−0.800, then were induced using IPTG. The following concentrations were tested: 0mM (no IPTG, this served as our negative control), 0.5mM IPTG, and 1mM. We had three test tubes set up for each concentration, each test tube served as a time trial for our harvesting time. Nine test tubes were set up in total, 1mL was harvested from each tube after three hours, four hours and overnight.
Figure F-02. Determination of optimal growth conditions. Small scale cultures were grown to determine conditions for maximum Flappase expression. Each sample set was harvested at a different time post-induction with IPTG. Sample set A, was harvested after 3 hours, sample set B after 4 hours, and sample set C was left to incubate overnight.
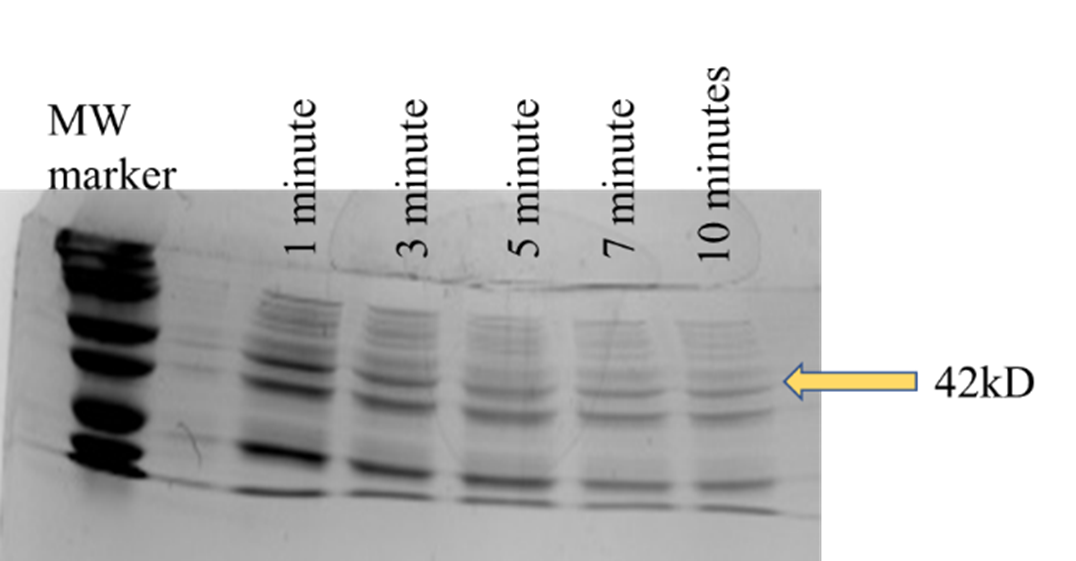
Cells were harvested using high speed centrifugation and washed with PBS buffer twice in order to ensure most of the cell debris was discarded. Then, we proceeded to lyse the cells via sonication. We conducted another set of experiments to ensure complete lysis. The cells were lysed via sonication for a total of 1 min, 3 mins, 5 mins, 7 mins and 10 mins. After sonication, the samples were subjected to SDS-PAGE and the gel was stained with Coomassie (Figure F-03). The one-minute sonication sample yielded very strong bands at ~42kD, which is the size of the desired Flappase construct. Not only are the bands the correct size, but they are darkest in color which means there is a high protein concentration present. The samples that were sonicated longer also show strong bands at the correct size. This tells us that only 1 minute of sonication was required to achieve complete lysis. We opted for the 3-minute sonication time to be sure that cells would be lysed, regardless of the viscosity of the samples.
Figure F-03. Optimization of cell lysis by sonication. SDS-PAGE gel showing the results off lysing cells using 1, 3, 5, 7, and 10 minutes of sonication time. All samples show a band at 42kD, the size of Flappase. No benefit was seen with respect to total protein yield beyond one minute.
The induction time trial samples were imaged on an SDS-PAGE gel in order to visualize which set of conditions is ideal for the highest yield of the Flappase protein. The optimal conditions for expression and induction resulted in 1mM IPTG harvested three hours post induction.
The western blot indicates that our inducible promoter in the Flappase construct is functional, but it is very weak. The faint markings in each lane show that the inducible promoter is producing the protein at all concentration levels. Only the overnight samples showed significant dose-dependent induction of Flappase. At the 1mM IPTG induction level with overnight incubation, we see the darkest line in the Western blot, and this shows that this sample contained a much higher concentration of the protein of interest.
Figure F-04. Western blot of induction time trials. All samples were lysed for 3 minutes; total protein loaded was the same in each lane. The His-tagged Flappase protein was visualized with an anti-His antibody. The yield of Flappase is much higher when induced with 1mM IPTG and left to incubate overnight.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 311
Illegal BglII site found at 704 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 216
| None |