Part:BBa_K3151008
Lac + yhjH PDE
This part utilizes the yhjH cyclic-di-GMP phosphodiesterase (BBa_K861090), and improves upon the Tec Chihuahua composite part (BBa_K2471001) by adding an inducible Lac promoter (BBa_R0010) and double terminator (BBa_B0015).
Usage and Biology
The native activity of cyclic-di-GMP as a secondary messenger in bacteria is primarily to regulate the transition between motile and sessile biofilm forming behaviour. Synthesis of cyclic-di-GMP itself is regulated by the stationary-phase sigma factor RpoS (σS), which when induced results in an increase in cyclic-di-GMP concentration, and subsequent downstream increase in the production of cellulose and curly fimbriae, the primary components of Escherichia coli biofilm.
Cyclic-di-GMP is synthesised by diguanylate cyclases, and degraded by phosphodiesterases. Many enzymes which perform these functions are capable of both synthesis and degradation of cyclic-di-GMP, however the yhjH phosphodiesterase is incapable of synthesis. Combined with the Lac promoter and double terminator, this Biopart allows for controlled expression of phosphodiesterase activity in a wide variety of E. coli strains such as DH5a, Nissle 1917 and BL21(DE3).
When combined with a cyclic-di-GMP riboswitch, such as BBa_K3151009, this phosphodiesterase can be used to control the expression of reporter genes through modulating the intracellular concentration of cyclic-di-GMP.
Characterisation
As intracellular cyclic-di-GMP concentration is directly correlated with biofilm formation[1], we can utilise the biofilm forming capability of the transformants as a proxy for intracellular cyclic-di-GMP concentration and phosphodiesterase activity. Nissle 1917 E. coli cells transformed with this Biopart were spotted onto LB agar plates supplemented with Congo Red and Coomassie Blue[2], and the cells transformed with the construct containing the promoter and terminator sequences produced significantly less cellulose than cells transformed with the construct without the promoter and terminator, as well as parental Nissle 1917 cells (Fig. 1), indicating significant phosphodiesterase activity within the cell.

Figure 1: E. coli Nissle 1917 cells transformed with the yhjH phosphodiesterase with (A) and without (B) promoter and terminator, as well as negative control (C). Cells were spotted onto LB agar plates supplemented with Congo Red and Coomassie Blue, and visualised with Blue Light LEDs. Nissle 1917 was selected as it produces significantly higher levels of cellulose in its biofilm. The red colour observed in (B) and (C) is the Congo Red staining the cellulose.
When this Biopart is combined with a cyclic-di-GMP riboswitch + eGFP reporter [BBa_K3151029] & [BBa_K3151030] (Fig. 3), it reduces the inhibition of eGFP transcription compared with the riboswitch + eGFP construct by itself [BBa_K3151011] & [BBa_K3151002] (Fig. 2). This is due to a decrease in cyclic-di-GMP concentration, which binds to the aptamer sequence of the riboswitch which terminates transcription.
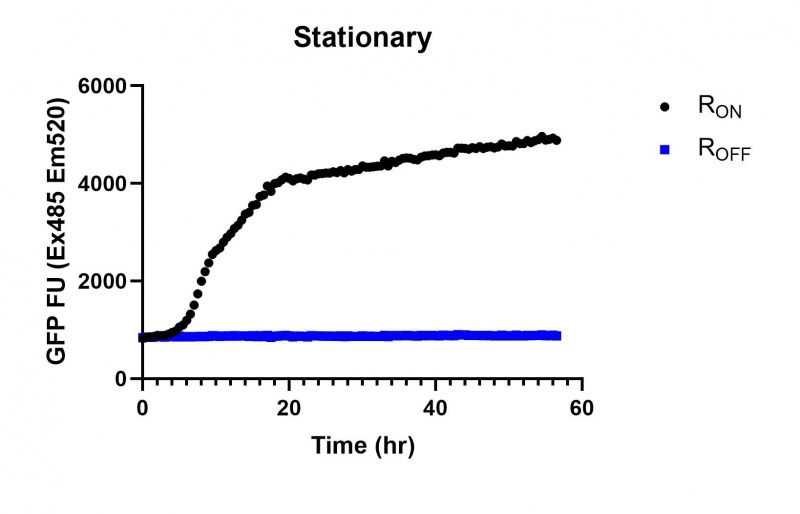
Figure 2: GFP fluorescence assay performed on cells transformed with RON [BBa_K3151011] and ROFF [BBa_K3151002]. Samples were measured with the BMG Pherastar plate reader to measure eGFP (Ex485 nm Em520 nm) and OD (600 nm).

Figure 3: GFP fluorescence assay performed on cells transformed with yhjH + RON [BBa_K3151029] and yhjH + ROFF [BBa_K3151030]. Samples were measured with the BMG Pherastar plate reader to measure eGFP (Ex485 nm Em520 nm) and OD (600 nm).
Improvement
When compared with the Tec Chihuahua composite part (BBa_K2471001), ours has the distinct advantage of being able to expressed in cells which lack T7 RNA polymerase. When our device was transformed into the E. coli strain Nissle 1917, the transformants exhibited a significant decrease cellulose biosynthesis (Fig. 4C) compared with the negative control Nissle 1917 cells (Fig. 4A), demonstrating highly active phosphodiesterase activity. Cells transformed with Tec Chihuahua's device (BBa_K2471001) show no significant decrease in cellulose biosynthesis (Fig. 4B) when compared with the negative control Nissle 1917 cells (Fig. 4A), suggesting no active phosphodiesterase activity.

Figure 4: Spot Assay of the negative control Nissle 1917 (A), Nissle 1917 with T7+yhjH (B), and Nissle 1917 with Lac+yhjH (induced with 1mM IPTG) (C). Plate observed under blue light to enhance visualisation of Congo Red-stained cellulose.
References:
- Weber H, Pesavento C, Possling A, Tischendorf G, & Hengge R. Cyclic‐di‐GMP‐mediated signalling within the σS network of Escherichia coli. Molecular microbiology. 2006 Nov;62(4):1014-34.
- Cimdins A, Simm R, Li F, Lüthje P, Thorell K, Sjöling Å, Brauner A, Römling U. Alterations of c‐di‐GMP turnover proteins modulate semi‐constitutive rdar biofilm formation in commensal and uropathogenic Escherichia coli. MicrobiologyOpen. 2017 Oct;6(5):e00508.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |
