Part:BBa_K3121000
Trigger Factor of P.haloplanktis
At low temperatures, the usual rate-limiting step for efficient growth is protein folding. In fact, the regular chaperone systems, for instance, the GroEL/ES are also faced with significant challenges at colder conditions, which severely hamper their activity. This leads to a dramatic decrease in bacterial growth and proliferation. The TF, in essence, is a cold-active chaperone, which is significantly upregulated at low temperatures. This is especially helpful because unlike standard chaperones, the TF has a very unique operational mechanism - it requires near-zero temperatures to stably bind unfolded polypeptides. Under cold conditions, the TF will find almost all the newly synthesized polypeptides, and thereby rescue the chaperone function of the cold-inactivated GroEL/ES (and similar chaperone complexes like the DnaK). Additionally, the TF is a monomeric chaperone unit, which has considerable structural flexibility in order to compensate for the reduced molecular motions at lower temperatures. Interestingly enough, the TF is not an essential gene under mesophilic growth conditions - indicating a specialized adaptive role. Moreover, the niche (yet indispensable) requirement of TF is underscored by the fact that the other two major chaperone systems - GroEL/ES and DnaK are significantly downregulated, as well as inactivated, under cold conditions - thereby making the TF system the only chaperon in this scenario.
Usage and Biology
In Pseudoalteromonas haloplanktis, the trigger factor is the first molecular chaperone interacting with virtually all newly synthesized polypeptides on the ribosome and also possesses a peptidyl-prolyl cis-trans isomerase activity. It acts as a chaperone by maintaining the newly synthesized protein in an open conformation. It has been proposed that the psychrophilic trigger factor rescues the chaperone function as both DnaK and GroEL (the major bacterial chaperones but also heat-shock proteins) are downregulated at 4°C.
Results
Immediately after synthesizing the TF we had to transform it into the appropriate cells. Once transformed, plasmid extraction was performed and the gel was obtained as may be seen in below.
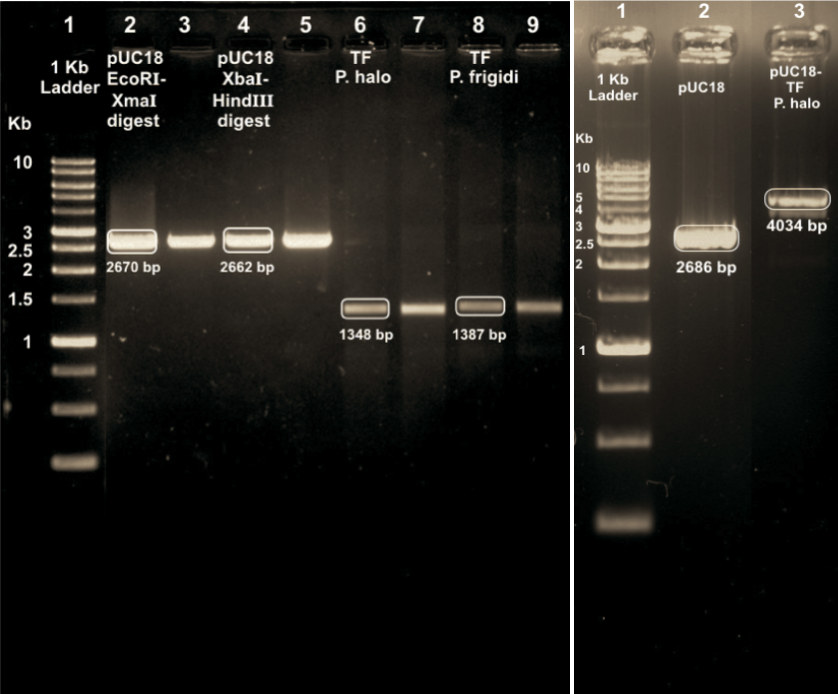
Post extraction, the plasmid was employed to transform cells, and its effect on cell growth was observed throughout the log phase. Our literature survey and IHP had helped us understand that optimal yield of different proteins is subject to different growth conditions - hence it was necessary for us to perform growth curve analysis and observe the effect of the Trigger Factor's induction on cell growth. The observations obtained for the same are given below. The temperatures chosen were 37C (as a model for optimal conditions) and 18C (as a model for suboptimal conditions). While we ideally would have liked to achieve even lower temperatures (as our model clearly predicts that TF could survive near 0C-like conditions), we were most unfortunately limited by the available infrastructural facilities.


In order to confirm the above results, we ran an SDS page of the whole cell lysate for the induced trigger factor. A distinct band was seen at 47.5kDa which is the weight of the protein. The gel image is seen below

| None |
