Part:BBa_K2959007
Expressible Wheat antimicrobial peptide 1b
This composite part consists of a lacI regulated promoter, ribosome binding site, a coding sequence for WAMP1b as a fusion protein with a 6x His-Tag, and a double terminator. This construct allows the expression of WAMP1b, an antifungal peptide from Triticum kiharae seeds, in E. coli. Expression can be positively regulated by the addition of IPTG or lactose thanks to the lacl regulated promoter. The part is designed to code for a fusion protein of WAMP1b with a polyhistidine tag (6x His-Tag) at its N-terminus for purification by immobilized metal affinity chromatography.
Usage and Biology
WAMP1b is an antimicrobial peptide from Triticum kiharae seeds. This peptide consists of 116 amino acids with a molecular weight of 11.5 kDa. Among these aminoacids, 10 cysteines are included, which form 5 nonconsecutive disulfide bonds C38-C53, C47-C59, C50-C78, C52-C66, and C71-C75. This composition makes it a highly stable molecule.1, 3
Hevein-like peptides, such as WAMP1b, contain a chitin binding domain as a structural motif of 35 amino acids with specific cysteine and glycine residues. Given this, the peptide is able to successfully bind to chitin, acting as a plant defense mechanism against fungi and certain insects3.
WAMP1b also inhibits fungalysin Fv-cmp, a protease produced by Fusarium fungi as a counterattack mechanism to plantās defenses, that cleaves chitinases. By inhibiting this protease, WAMP1b acts as an additional defense mechanisms against fungal pathogens. It has also been observed that this peptide directly inhibits hyphal elongation.4
In an investigation done by Slavokhotova et al., (2014), WAMP1b was tested against various fungi, and it was proved effective against against F. verticillioides with an IC50 of 2.7 Āµg/ml.
Characterization of Expressible Wheat antimicrobial peptide 1b
Our DNA sequence WAMP1b was synthesized by IDTĀ®ļø with the Biobrick prefix and suffix flanking the composite part. This made possible the correct digestion with restriction enzymes EcoRI-HF and PstI. After the digestion, ligation was performed with T7 ligase in order to place our construct into the pSB1C3 linearized backbone with chloramphenicol resistance, which was previously digested with the same restriction enzymes. Using the SnapGeneĀ®ļø software, we could model our ligated expression plasmid, and the final part resulted in a sequence of 2,590 bp. Thereupon,Escherichia coli SHuffle was transformed by heat shock for following antibiotic selection of clones.

The next step was to amplify our BioBrick sequence through colony PCR performed upon our transformed cells to confirm the presence of our expression plasmid inside of our chassis. With the help of the specific forward Biobrick prefix [BBa_G1004] and the specific reverse Biobrick suffix [BBa_G1005], we were able to amplify our sequence exclusively. Through an agarose gel we confirmed the correct transformation. The PCR action from SnapGeneĀ®ļø was used to predict the size of the amplified sequence which resulted in a size of 579 bp.


Protein production
IPTG Induction and Extraction
Following the construction of the BioBrick, it was necessary to induce protein production. Production of WAMP1b was possible under Lacl promoter when induced with 0.2 or 0.4 mM IPTG at 30Ā°C and 225 rpm. This was followed by protein extraction by lysis solution to which lysozyme was added in order to obtain our soluble peptides.
Antifungal Assay
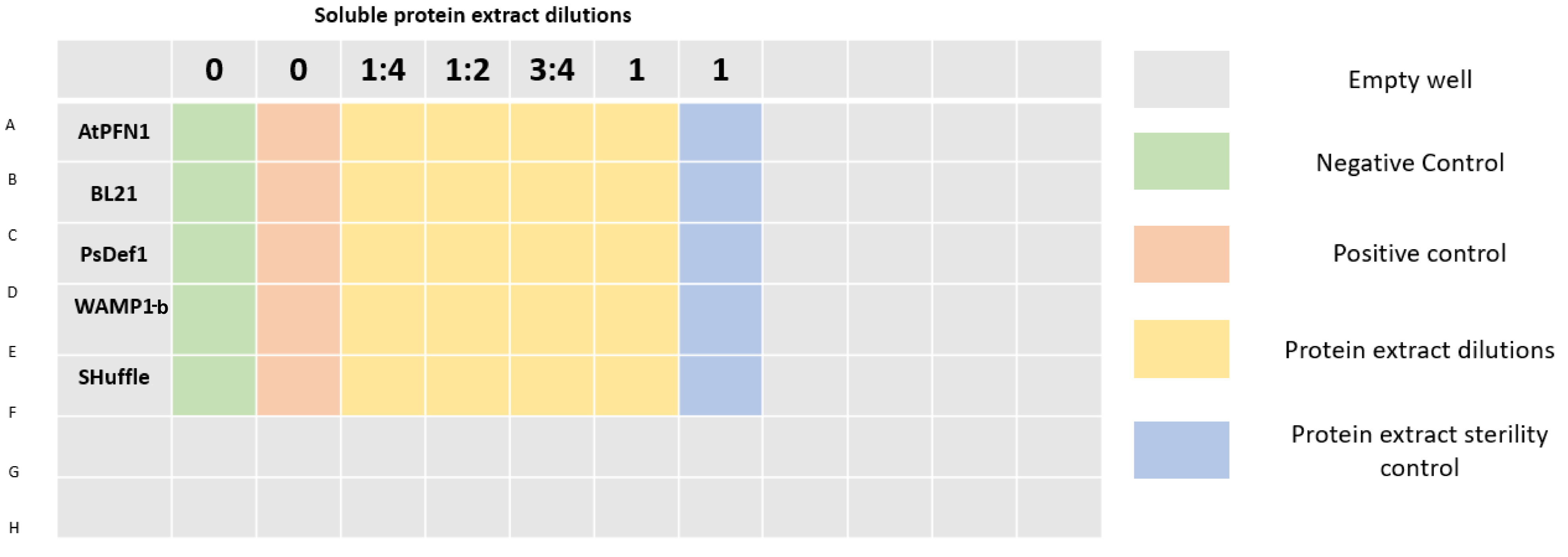
In order to test the antifungal activity of our peptides as well as prove their viability as a mechanism to inhibit Verticillium dahliae, an antifungal susceptibility test on a 96 well plate was carried out by measuring absorbance at 405 nm, wavelength used in standardized protocols to measure growth of filamentous fungi5. Due to a lack of time, we couldnāt reach the experimental stage of the project of peptide purification, so experiments were made using soluble protein extracts from our transformed cellsā lysates. Different dilutions of the extracts were prepared which were applied to a spore suspension of V. dahliae. Dilutions of the extracts of untransformed cells were used as controls to prove that inhibition was the result of the peptides and not any other protein contained within the extract. Soluble proteins from E. coli SHuffle were used as control for the WAMP1b extract. Concentrations of extracts with peptides were equalized to their controls. Table 1 details the concentrations of each dilution.
| Dilution | WAMP1b(mg/mL) | SHuffle control (mg/mL) |
|---|---|---|
| 1 (undiluted) | 3.7402 | 3.74 |
| 3:4 | 2.8052 | 2.805 |
| 1:2 | 1.8701 | 1.87 |
| 1:4 | 0.9351 | 0.935 |
96 well plates were prepared as shown in Figure 3. A final volume of 200 Ī¼L was completed in each well by mixing protein extract, sterile distilled water (to achieve desired concentrations), potato dextrose broth, and a spore suspension of V. dahliae with a final concentration of 2x104 spores/mL per well. Plates were incubated at 25Ā°C.
Absorbance readings at 405 nm were performed in a Varioskan Lux 3020-231 microplate reader 1 and 24 hours after plate preparation. After analyzing the results, a growth rate percentage was estimated for every evaluated sample by using the following formula:
Growth rate = ((A1 - A0)/A0) x 100
Where:
A1 = Absorbance after 24 hours.
A0 = Absorbance after 1 hour.
Results
Results generated by the previous formula correspond to the percentage in which absorbance increased in each well compared to the initial reading. The results are summarized in figures 4. As the figure show, an outstanding difference in growth values exists among the positive growth controls without any kind of protein extract, the untransformed strain extract controls, and the growth of the fungus with different peptide concentrations, was noted. The positive control was estimated to grow at a rate of 40.36% after 24 h, on the other hand, the lowest concentrations of added protein extracts clearly showed a decreasing behavior in growth rates. In comparison, growth rates from wells containing protein extracts of untransformed cells do not behave this way, meaning that WAMP1b had a degree of inhibition against V. dahliae, even in crude extract.
In order to better comprehend the results, growth rates were used to estimate the percentage of inhibition using the following formula:
I = 100 - (Gp/Gv) x 100
Where:
I = Inhibition %
Gp = Growth rate of V. dahliae treated with extract with peptides.
Gv = Growth rate of V. dahliae positive control.
During the evaluation of WAMP1b, untransformed protein extracts seemed to increase the fungusā growth in contrast with the extract containing the peptide. However, in figure 4, inhibition caused by WAMP1b can be observable in the two lowest dilutions, presenting high inhibition percentages of 97.52% and 81.54% for dilutions 1:2 and 1:4, respectively. These reductions in growth rate helped to characterize the peptideās reported antifungal activity.
Discussion
When evaluating the results, we looked for an explanation for the inhibitory effect of the peptide occuring at lower concentrations of crude extract. A common occurrence in microplates assays is the precipitation of the inhibitory components, which leads to inconsistent inhibition values.6 Peptides tend to precipitate at certain concentrations, depending on their composition, solubility and even the buffer or medium theyāre diluted in.6, 7 Given that we have almost no concrete information about our peptides, and that we saw ourselves forced to use crude extract, precipitation at the highest concentrations we used is a feasible possibility. It has been remarked in literature that this incidence makes it almost impossible to determine the MIC (Minimal Inhibitory Concentration) through this method.6
The precipitation of our peptides could have affected the optic density measurements causing variations that altered the overall results.7 Or, most likely, the precipitation altered the inhibitory effect of our peptides at high concentrations. This is a common occurrence, and recommendations can be found through literature, research papers, and protocol manuals.8
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 261
References
1.Dubovskii, P. V., Vassilevski, A. A., Slavokhotova, A. A., Odintsova, T. I., Grishin, E. V., Egorov, T. A., & Arseniev, A. S. (2011). Solution structure of a defense peptide from wheat with a 10-cysteine motif. Biochemical and Biophysical Research Communications, 411(1), 14ā18. doi: 10.1016/j.bbrc.2011.06.058
2. Istomina, E. A., Slavokhotova, A. A., Korostyleva, T. V., Semina, Y. V., Shcherbakova, L. A., Pukhalskij, V. A., & Odintsova, T. I. (2017). Genes encoding hevein-like antimicrobial peptides WAMPs in the species of the genus Aegilops L. Russian Journal of Genetics, 53(12), 1320ā1327. doi: 10.1134/s1022795417120043
3. Odintsova, T. I., Vassilevski, A. A., Slavokhotova, A. A., Musolyamov, A. K., Finkina, E. I., Khadeeva, N. V., ā¦ Egorov, T. A. (2009). A novel antifungal hevein-type peptide fromTriticumākiharaeseeds with a unique 10-cysteine motif. FEBS Journal, 276(15), 4266ā4275. doi: 10.1111/j.1742-4658.2009.07135.x
4. Slavokhotova, A. A., Naumann, T. A., Price, N. P. J., Rogozhin, E. A., Andreev, Y. A., Vassilevski, A. A., & Odintsova, T. I. (2014). Novel mode of action of plant defense peptides - hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS Journal, 281(20), 4754ā4764. doi: 10.1111/febs.13015
5. Schwalbe, R., Steele-Moore, L., & Goodwin, A. C. (2007). Antimicrobial susceptibility testing protocols. Crc Press.
6. Eloff, J. (1998). A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Medica, 64(08), 711ā713. doi:10.1055/s-2006-957563
7. Morishige, H., Mano, Y., Oguri, T., & Furuya, N. (2012). Comparison of four reading methods of broth microdilution based on the Clinical and Laboratory Standards Institute M27-A3 method for Candida spp. THE JAPANESE JOURNAL OF ANTIBIOTICS , 65(5), 335ā347. Retrieved from http://jja-contents.wdc-jp.com/pdf/JJA65/65-5/65-5_335-347.pdf
8. Berditsch, M. (2012). Two-fold Broth Microdilution Method for Determination of MIC . Institute for Bio and Geosciences. Retrieved from http://www.ibg.kit.edu/nmr/downloads/MICprotocoll_30_Jan2012.pdf
| None |