Coding
Part:BBa_K2926079
Designed by: Ina Schmitt Group: iGEM19_Bielefeld-CeBiTec (2019-10-15)
Kanamycin resistance protein Part 2
hgs
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 28
Ampicillin
Another antibiotic used to prevent bacterial infections poses ampicillin. Similar to other antibiotics, it can be administrated via mouth and injection into a muscle. It is classified under the category of bacteriolytic antibiotics and can penetrate gram-positive and gram-negative bacteria. It is responsible for the inhibition of transpeptidase, that is responsible for the cell wall of the bacteria by inhibiting the final cell wall synthesis in binary fission (Ampicillin Monograph for Professionals, 2018)
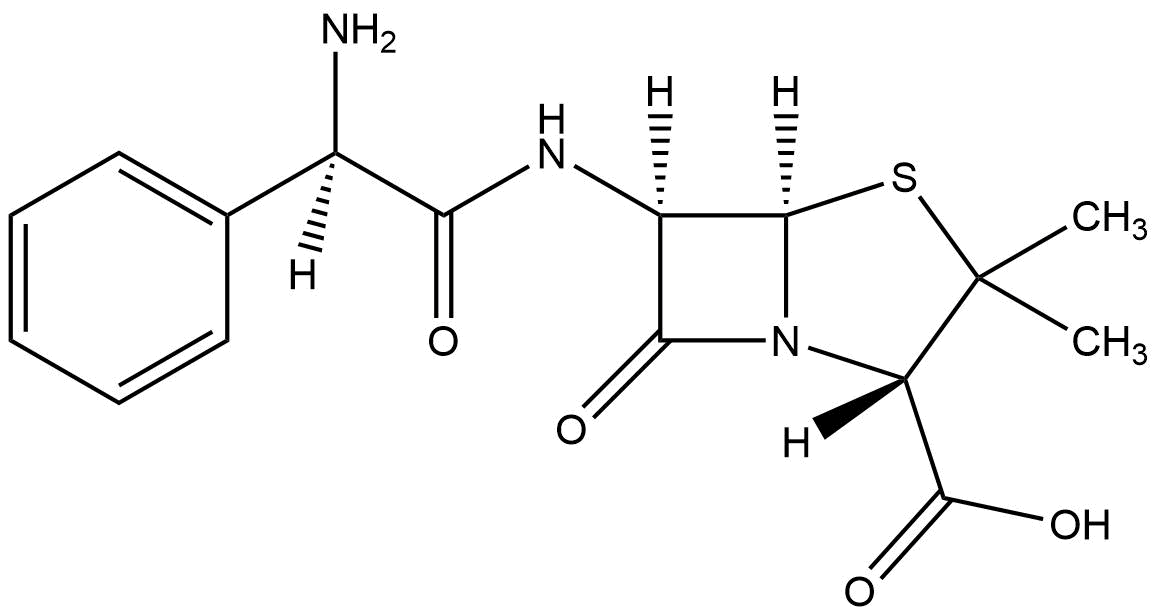
Resistance Ampicillin
Beta-lactamases are enzymes that bacteria produce to defend themselves against β-lactam antibiotics. The most common lactamases are the class A enzymes, such as the clinically significant TEM-1 lactamase. (Waley, 1992). It provides antibiotic resistance by breaking the antibiotics structure. All these antibiotics all have a common element in their molecular structure: a four-atom ring known as a β-lactam. Through hydrolysis, the enzyme breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.
Chloramphenicol
A commonly used antibiotic for bacterial infections is chloramphenicol (Chloramphenicol Monograph for Professionals, 2019). It was isolated from Streptomyces venezuelae in 1947 and was the first artificially produced antibiotic (Pongs O.,1979). Once it passes the cell membrane it has a bacteriolytic effect. It binds to specific residues of the 23S rRNA of the 50S ribosomal subunit, that hampers the substrate binding in the ribosome (Chloramphenicol - Infectious Diseases, 2019) (Wolfe, A. D., 1965). Chloramphenicol is often one of the ingredients in eye ointments for the treatment of conjunctivitis (Edwards 2009). In some other cases it can be administered by mouth or vein injection (Rosenfield, Logan, & Edwards, 2009). Furthermore, it is used to treat hazardous bacterial infections like plaque, cholera, MRSA or typhoid fever (Ingebrigtsen 2017).
[edit]
Categories
Parameters
| None |
