Part:BBa_K2715003
Clostridial promoter Pcdi_tcdB with its native 5' UTR and RBS, driving a GFP reporter
Usage and Biology
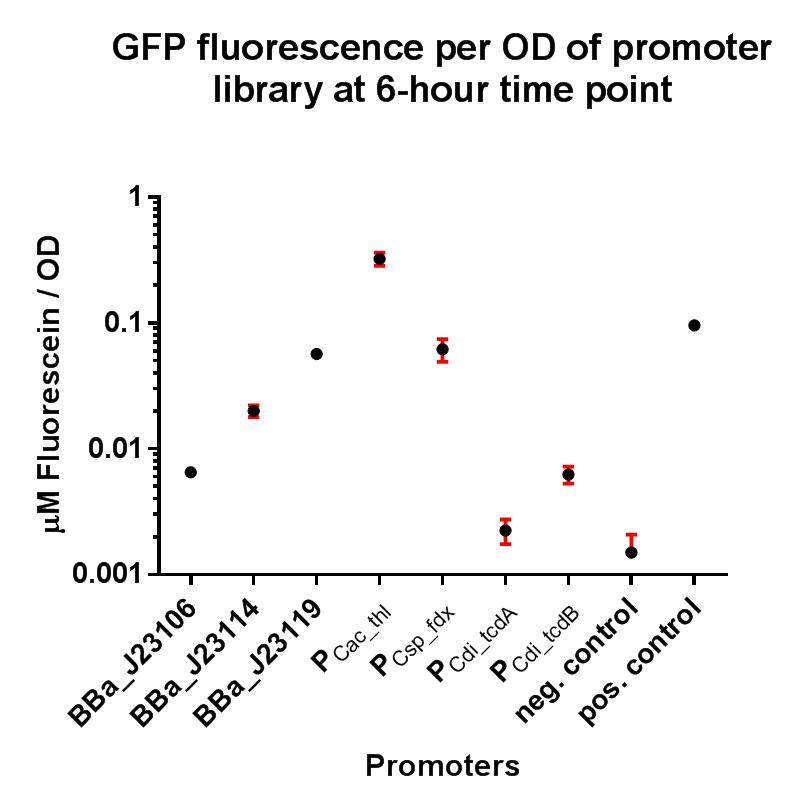
Our project required the use of strong constitutive promoters which would function well in the non-model Gram-positive organism Clostridium difficile. We also wanted to establish whether these promoters functioned in E. coli, as this could have implications for cloning stages and vector assembly when trying to build constructs containing potentially toxic genes. In order to put the strength of these promoters into context, we decided upon a GFP fluorescence assay using the iGEM Interlab calibration curves, and also to compare their strengths to the Interlab positive and negative controls. This promoter was taken directly from the organism in which we wished to demonstrate our novel methods (C. difficile) for controlling toxin production, as we wanted to gain a greater understanding of the level of expression from this promoter in the wild type organism. We decided to explore its expression levels in E. coli, as it has been shown in previous research to be under the regulation of an alternative sigma factor encoded by tcdR, present in the C. difficile genome, and therefore we expected to observe reduced expression in E. coli . The variant of this composite part used for testing expression levels in C. difficile can be found here BBa_K2715027.
Characterisation
This composite part is composed of the native clostridial promoter driving expression of the toxin gene tcdB, sub-divided into the promoter region itself BBa_K2715013, the 5’ UTR region BBa_K2715023, and the RBS BBa_K2715024, driving expression of GFP taken from BBa_E0040. The construct is part of a family of composite parts driving expression of the GFP gene and were all characterised in the same plasmid backbone and in parallel in a fluorescence assay, the results of which can be seen below. The positive and negative controls are parts BBa_I20270 and BBa_R0040 respectively, used in the Interlab 2018 study.
The composite parts tested in this assay under the same conditions using a range of alternative promoters are as follows:
BBa_K2715106
BBa_K2715114
BBa_K2715119
BBa_K2715001
BBa_K2715002
BBa_K2715003
BBa_K2715004
These composite parts were assembled in the shuttle cloning vector pMTL84151, and characterised in E. coli within this plasmid. Additionally these promoters were characterised in C. difficile using the gusA biobrick BBa_K330002 as a reporter gene in place of GFP, as GFP requires oxygen in order to function, and C. difficile is an anaerobic organism. The gusA containing composites used to assay the promoter activities in C. difficile are listed below.
BBa_K2715025
BBa_K2715026
BBa_K2715027
BBa_K2715028
BBa_K2715029
BBa_K2715030
BBa_K2715031
The plasmid used for this characterisation in E. coli is displayed below.
Conclusions
This composite part has enabled a standardised characterisation of the native promoter driving the tcdB gene and its strength can be quantified in E. coli using the iGEM 2018 interlab units of fluorescence. It has been demonstrated in this assay to be fairly weakly expressed, suggesting that it may indeed by subject to regulation by an alternative sigma factor, or perhaps it may just be that this clostridial promoter is poorly expressed in the Gram-negative organism E. coli.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 959
Illegal SapI.rc site found at 139
References
Heap, J.T., Pennington, O.J., Cartman, S.T. and Minton, N.P., 2009. A modular system for Clostridium shuttle plasmids. Journal of microbiological methods, 78(1), pp.79-85.
Davis, D.F., Ward, W.W. and Cutler, M.W., 1994. Posttranslational chromophore formation in recombinant GFP from E. coli requires oxygen. In Bioluminescence and Chemiluminescence: Fundamentals and Applied Aspects. Proceedings of the 8th International Symposium on Bioluminescence and Chemiluminescence, Cambridge. Wiley, New York, NY (pp. 569-599).
Chiu, N.H. and Watson, A.L., 2017. Measuring β‐Galactosidase Activity in Gram‐Positive Bacteria Using a Whole‐Cell Assay with MUG as a Fluorescent Reporter. Current protocols in toxicology, 74(1), pp.4-44.
| None |