Part:BBa_K2665020
SED1 secretion signal-FgBF3-ddFLN4-FLAG tag-ybbr tag-Stalk Region-SED1 anchoring domain
Description
This part contains various parts. This part is designed for surface-display of FgβF3 on yeast cell wall and aggregation with yeast that has SdrG on its surface. Part that includes SdrG is BBa_K2665021
Fgβ is b chain of human fibrinogen. Fgβ combines with SdrG very strongly. This bonding is comparable to covalent bond in strength. [1] We uses F3 domain of Fgβ BBa_K2665018 for this part because it is essential and enough to combine with SdrG.
This part also includes ddFLN4 BBa_K2665024 . In the paper, description of ddFLN4 is not enough, but it seems to be used for assay of bond strength. The ybbr-tag BBa_K2665023 is also included in this part. These part was included in design of the paper, so we leaved these part just in case.
FLAG-tag BBa_K2665028 was fused for several assays of aggregation. Detail of assay in the experience page.
We use SED1 secretion signal and SED1 anchoring domain in order to display FgβF3 on yeast cell wall. More detail about these parts are in the pages of BBa_K2665025 and BBa_K2665026
“Stalk Region” BBa_K2665022 is a very long linker. In order to combine yeasts, we have to provide enough room between yeasts. We referred the research that display of nanobodies on yeast cell wall. [2]
Characterization
Yeast displaying FgβF3 on the cell surface was captured with magnetic beads coated with anti-Flag antibody, and after washing with PBS, the bound cells were observed with a microscope.
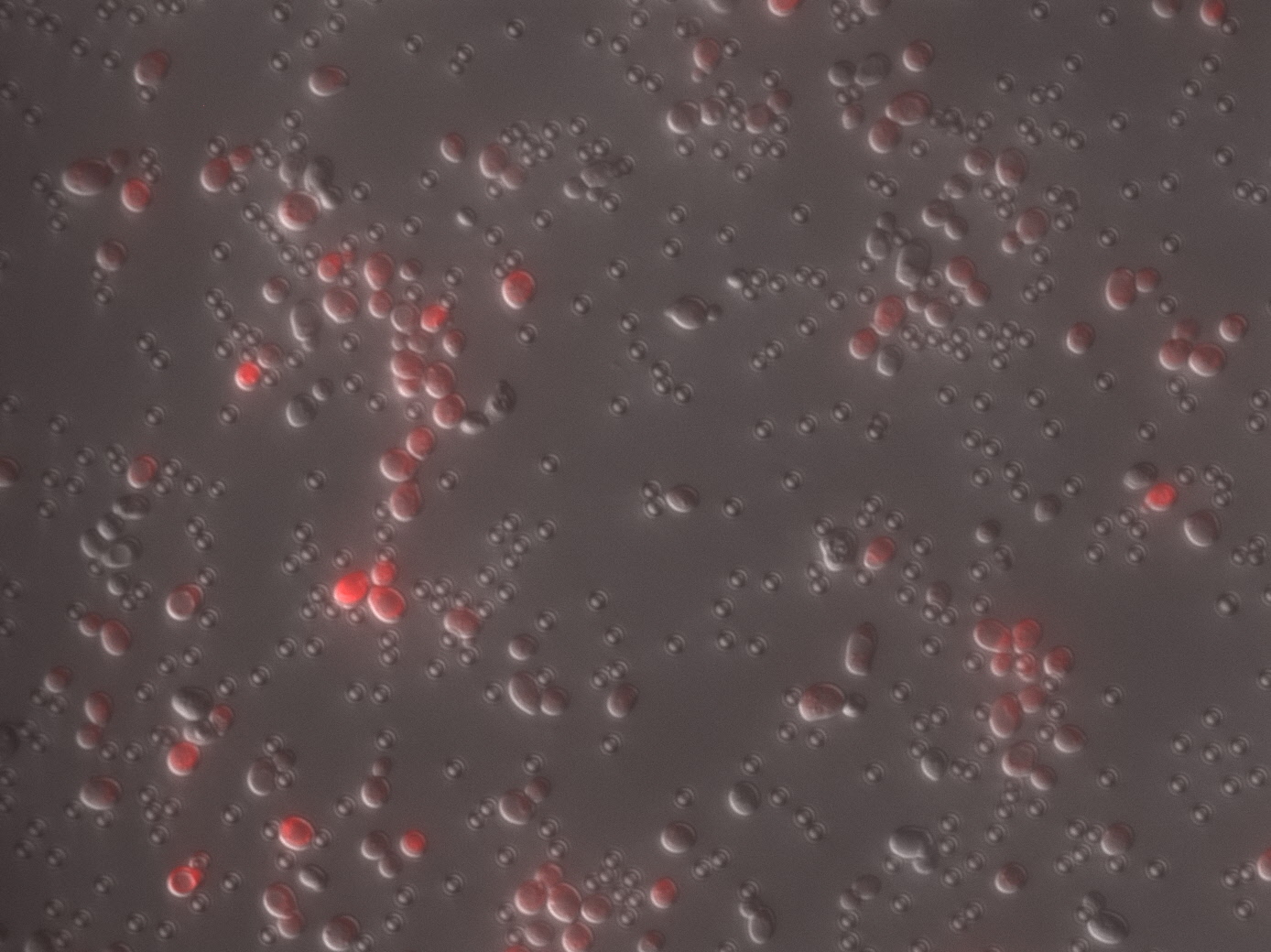
The results are shown in the Figure 1. As expected, yeast containing Fgβ (Flag) bound very well to anti-Flag antibody beads. It was observed that yeast with many RFP signals was bound to the washed beads. In contrast, when yeast displaying SdrG (6xHis) was used in the same manner, yeast with almost GFP signal did not settle. This indicates that anti-Flag antibody beads do not randomly precipitate yeast at all, but rather specifically recognize and settle the Flag sequence displayed on the cell surface. From the above results, the following was clarified.
(1) FgβF3 (Flag) was appropriately displayed on the surface of yeast cells by fusion with SED1 anchoring domain.
(2) Only specific yeasts could be immobilized on magnetic beads using the antigen placed on the surface.

In addition, mixture of Fgβ(FLAG) expressing cells and SdrG (His 6) expressing cells was captured with magnetic beads coated with anti-Flag antibody. Figure 2 shows significant decrease in number of captured cell compared to Figure 1. We cannot assume what is going on, but it may be consequent of interaction between Fgβ and SdrG.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 468
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 2809
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 624
Illegal SapI site found at 944
Illegal SapI site found at 2746
Illegal SapI.rc site found at 126
Reference
[1]L.Miles, K.Schulten, H.Gaub et al. (2018) "Molecular mechanism of extreme mechanostability in a pathogen adhesin" Science Vol.359 1527-1533
[2]C.Mcmahon, A.Baier, R.Pascolutti et al. (2018) "Yeast surface display platform for rapid discovery of conformationally selective nanobodies" Nature Structural & Molecular Biology Vol.25
| None |
