Part:BBa_K2596003
PpsbA2 from Synechococcus elongatus PCC 7942
This a high-light inducible promoter from cyanobacteria Synechococcus elongatus PCC 7942
Usage and Biology
PpsbA2 is a light-inducible promoter native to cyanobacteria. Specifically, it comes from our chassis organism S. elongatus PCC 7942. The promoter is activated by high light intensity or blue light, and deactivated by low light intensity or red light [1].
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
The promoter contains a conserved AT-box motif just upstream of the ribosome binding site that serves as the binding site for light-activated proteins. For the Biobrick, we kept most of the original promoter, including a crucial upstream element and the core promoter sequence. Our final Biobrick is 157 bp in length.
Theory: PsbA2 is a promoter that encodes for the D1 protein, a protein involved in photosystem II and which degrades faster (~every 20 minutes compared to every 5 hours) upon exposure to high light intensity. There are three promoters involved in synthesizing D1: PsbA1, PsbA2, and PsbA3. These promoters work differently in different strains of cyanobacteria; however, in S. elongatus PCC 7942, PsbA1 is transcribed under normal light conditions (~120 uM photons/m^2/s), while PsbA2 and PsbA3 are actively repressed [2].
Upon exposure to high light (~>500 uM photons/m^2/s), PsbA2 and PsbA3 are transcribed, while PsbA1 is repressed [3]. These properties make the promoter PsbA2 an attractive optogenetic biobrick for toggling the gene expression of proteins involved in the production of carbon-based products in cyanobacteria.
Luciferase Assays
Experimental Methods:
In order to test the expression of our promoters, cpc (BBa_K2596001), cpc560 (BBa_K2596006), idiA (BBa_K2596004), and psbA2 (BBa_K2596003), which were incorporated into Dr. Susan Golden’s vector pAM1414 using Gibson assembly, we conducted luciferase experiments. Following Dr. Golden’s procedure, we added 5 µL of decanal to 95 µL of cyanobacteria in each well to induce expression. The decanal acted as a substrate for the bacterial luciferase enzyme, but due to the toxicity, the cells ended up dying, so the data obtained represents the end static expression. For the standard expression experiments for nighttime and daytime, we plated 95 µL of cyanobacteria into a 96 well plate, added 5 µL of decanal, parafilmed the edges and left the plate for 15 minutes before measuring the luminescence in a plate reader. For the high light experiment to psbA2 promoter, we plated 95 µL of cyanobacteria to half of the wells and put them under at least 500 µE of high light in our incubator for 1 hour. Then, we added 95 µL of cyanobacteria not exposed to high light to the other half of the wells and added 5 µL of decanal to all of the wells. Then, we placed the well plate in a plate reader and measured the luminescence. For the iron repressible experiment to idiA promoter, we plated 95 µL of cyanobacteria to half of the wells and exposed them to 2.3 mM of iron chelating agent 2,2-dipyridyl for one hour. Then, we added 95 µL of cyanobacteria not exposed to the iron chelating agent to the other half of the wells and added 5 µL of decanal to all of the wells. We placed the well plate in a plate reader and measured the luminescence.
Results:
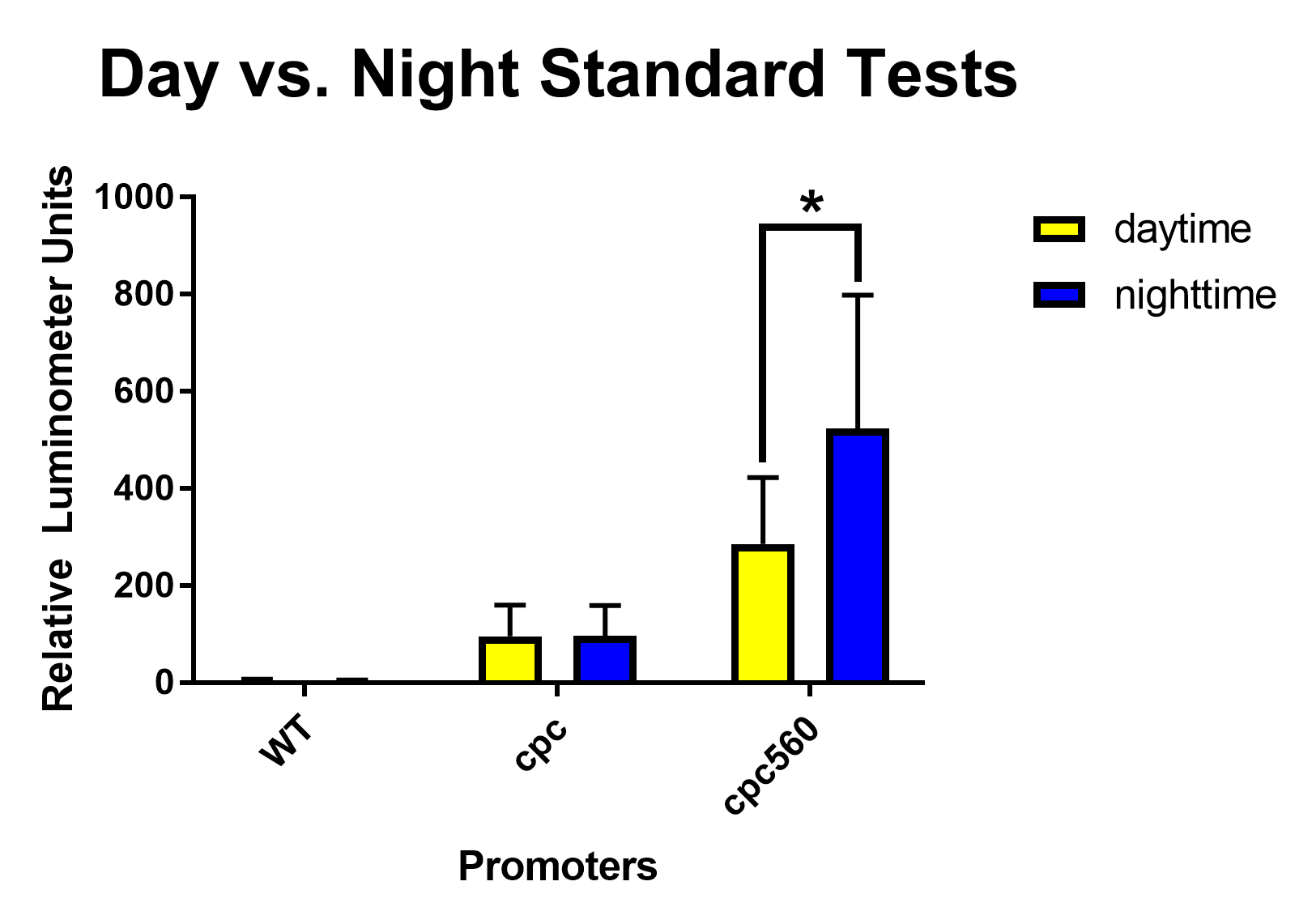
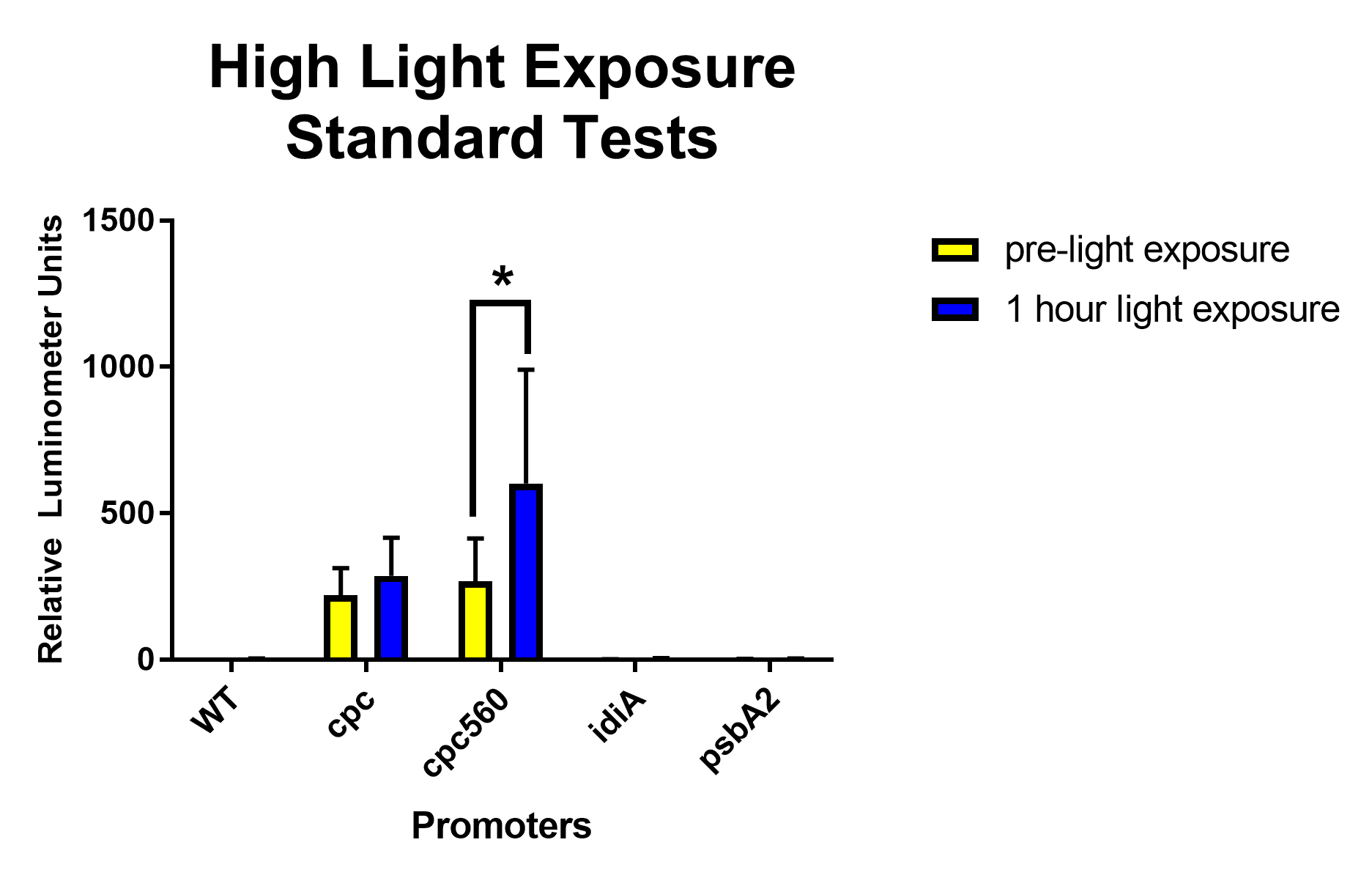
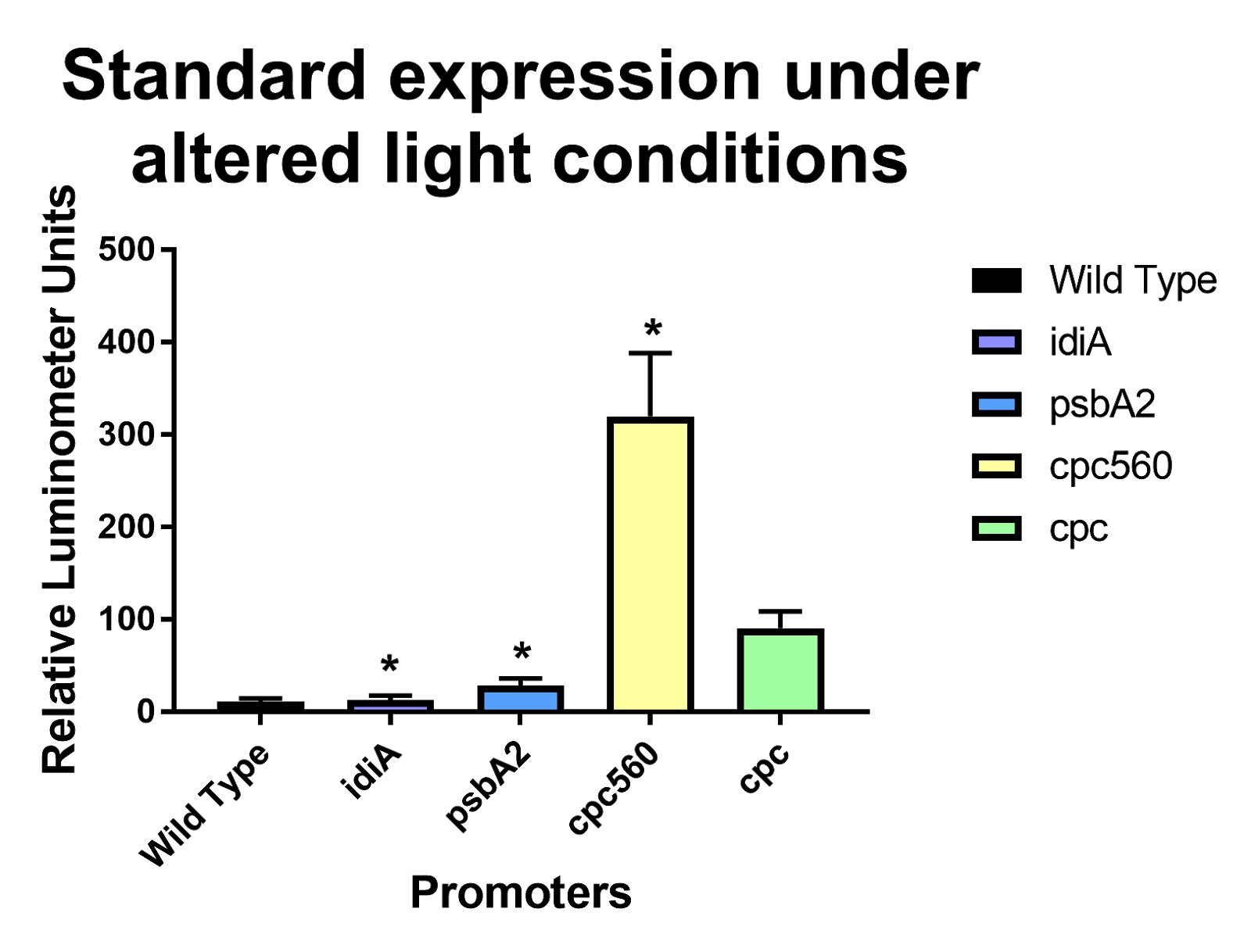
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, both cpc and cpc-560 showed significant differences for daytime expression compared to wild type [Figure 1]. Compared to cpc, cpc-560 had a significantly higher expression [Figure 1]. In comparison for day compared to night, cpc did not show any significant difference [Figure 2]. On the other hand, cpc-560 showed a significant difference between daytime and nighttime expression [Figure 2]. After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for daytime expression, compared to all other promoters and the wild type, cpc and cpc-560 had significant differences in expression [Figure 1]. For idiA and psbA2, they only had significant differences compared to cpc and cpc-560, and not to each other [Figure 1]. When exposed to highlight, idiA and psbA2 did not show any expression [Figure 3].
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for expression after iron repression, idiA, psbA2, and cpc-560 all showed a significant difference between before iron chelator reagent addition and after [Figure 4]. cpc’s insignificant difference could be attributed to the fact that there were only two cpc samples, as a result of insufficient cyanobacterial growth. The significant decrease in the expression of cpc-560, however, as a result of adding the iron chelator, indicates that the cells may have died from prolonged deficiency of ferrous ions, therefore that one hour may have been too long [Figure 4].
When conducting our ferrous standard expression experiments, we had just replenished the media with antibiotics and left the untreated cyanobacteria as well as the treated cyanobacteria in our biosafety cabinet, not expecting any major changes in expression due to the light exposure. However, psbA2 was expressed significantly more than wild type [Figure 5]. We propose that this may be due to replenishing antibiotics in the medium or from the stress of leaving them in the biosafety cabinet.
psbA2 activity in novel chassis S. elongatus UTEX 2973
IISER-Pune-India 2021:
psbA2 is a native promoter found in S. elongatus, among other cyanobacteria species. Though it is induced by higher light intensities, it effectively shows constitutive expression at constant light conditions [4]. The psbA2 promoter from the strain S. elongatus PCC 7942 and from S. elongatus UTEX 2973 share a 100% sequence identity (verified by NCBI BLAST search).
Sequence of psbA2 from S. elongatus UTEX 2973 [5]:
GAAAAGTCTGAAAGTTCTTTACAAAACTCAATCTGCTTGTTAGATTTTACTCAC GAGGCTATTAAGTCTCGTAAATAGTTCAACTAAGGACTCATCGCAAA
Shubin Li et al., characterised and compared the activity of various constitutive promoters in S. elongatus UTEX 2973, including psbA2, native to S. elongatus UTEX 2973 [5]. The characterisation was done at both high and low light intensities - 500 umol photons/m2/s and 50 umol photons/m2/s respectively - using the reporter gene lacZ, encoding beta-galactosidase.
The activity of beta-galactosidase was calculated using Miller value (Miller = 1000 × OD420 nm/(total volume of the cell culture × reaction time × OD750 nm of the cell)). Transcriptional expression of lacZ was measured using qRT-PCR.
Promoter-lacZ-trbcL constructs, flanked by Neutral Site 1 homology arms, were made on a pBR322 backbone vector (see fig. 6b). The pBR322 backbone contains an oriT/bom site, allowing it to be introduced into UTEX 2973 by conjugation. The Neutral Site 1 homology arms allow for genomic integration of the Promoter-lacZ-trbcL construct into the Neutral Site 1 on UTEX 2973's genome via homologous recombination following conjugation.
Constitutive promoters characterised in UTEX 2973 by Shubin Li et al.:
a. psbA1, psbA2, psbA3, cpcB1, cpcB2 from UTEX 2973
b. cpc560 and psbA2 from Synechocystis PCC 6803
c. lac from E. coli
d. trc, LlacO-1, psbA from the chloroplast of Amaranthus hybridus
Their results indicate that psbA2 is a fairly strong promoter at high light intensities.
Beta-glactosidase had a higher activity under psbA2 than commonly under used constitutive promoters psbA1, cpcB1, and cpcB2 (see fig. 6a) at high light intensity. However, the transcriptional expression of lacZ under psbA2 is lower than that of lacZ under cpcB1, cpc560, psbA1 and psbA3 promoters (see fig. 6a) at high light intensity.
psbA1 shows nearly a three-fold increase in strength under high light conditions when compared to low light conditions (see fig. 6c).

References:
[1] Tsinoremas NF1, Schaefer MR, Golden SS. "Blue and Red Light Reversibly Control psbA Expression in the Cyanobacterium Synechococcus sp. Strain PCC 7942.” J Biol Chem. 1994 Jun 10;269(23):16143-7.
[2] Mulo, Paula, Cosmin Sicora, and Eva-Mari Aro. “Cyanobacterial psbA Gene Family: Optimization of Oxygenic Photosynthesis.” Cellular and Molecular Life Sciences 66.23 (2009): 3697–3710. PMC. Web. 17 Oct. 2018. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2776144/
[3] RESHAM D. KULKARNI AND SUSAN S. GOLDEN. "Adaptation to High Light Intensity in Synechococcus sp. Strain PCC 7942: Regulation of Three psbA Genes and Two Forms of the D1 Protein." JOURNAL OF BACTERIOLOGY, Feb. 1994, P. 959-965
[4]Till, P., Toepel, J., Bühler, B. et al. Regulatory systems for gene expression control in cyanobacteria. Appl Microbiol Biotechnol 104, 1977–1991 (2020). https://doi.org/10.1007/s00253-019-10344-w
[5]Li, S., Sun, T., Xu, C., Chen, L., & Zhang, W. (2018). Development and optimization of genetic toolboxes for a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Metabolic engineering, 48, 163-174
//promoter
| biology | Synechococcus elongatus PCC 7942 |
| device_type | Inducible Promoter |