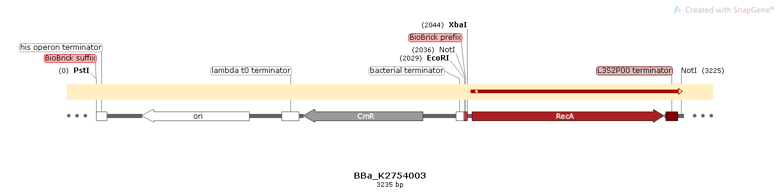
Part:BBa_K2457003
Standardized RecA coding sequence
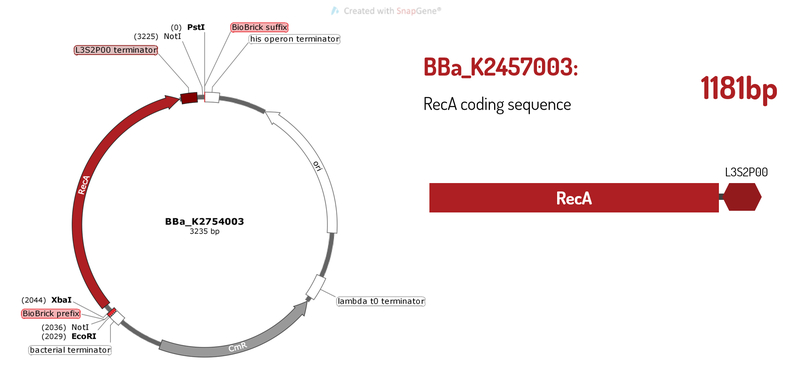
The BioBrick BBa_K2457003 is an essential building block part to constructions that will be involved in homologous recombination processes. It was developed by Amazonas_Brazil team in 2017 to be a CRISPeasy vector building block: a standard BioBrick vector to bacterial genome engineering. This part has the length of 1130bp and it is oriented in reverse direction, being composed by: the RecA coding sequence and the L3S2P00 transcription terminator.
Figure 1: BBa K2457003 circuit.
Usage and Biology
After the cleavage by Cas9 in the target sequence at a ribonucleoprotein complex with the guide RNA, blunt-end strands are originated. In SOS response, the endogenous RecBCD repair enzymes from the cell machinery degrade the blunt-end extremities in 5’ to 3’ direction, up to the chi site recognition. Then, their activity in 5’ to 3’ direction is finished and keeps going to 3’ strand, forming 3’ DNA single stick-ends strands. This segment is recognized by RecA, which builds a DNA-protein filament able to search for homologous template on undamaged DNA sequences, assisting on the polymerase invasion process and setting-up the Holliday Junction. This generates a crossover with the strands, leading to the recombination among the undamaged strand and the broken strand. Now let's analyze the functional scheme of this building block:
Assembly
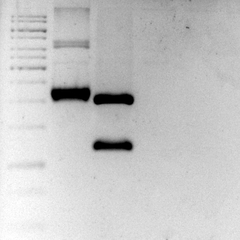
The sequence was ordered as gBlocks from Integrated DNA Technologies and it was assembled by restriction enzyme site.
Figure 2: Eletrophoretic profile of restriction digestion of BBa_K2457003.
Characterization
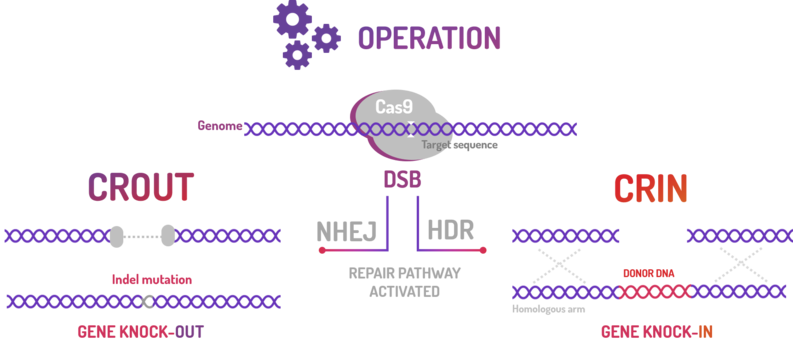
BBa_K2457003 was used by Amazonas_Brazil team in one method based on standard BioBricks modules for bacterial genome engineering mediated by CRISPR-Cas9 machinery: the CRISPeasys. The approaches constructed were 1) for non-homologous end joining (NHEJ) repair pathway, the CRISPeasy-out method (or crOUT), referring to knock-out and the 2) for homologous directed repair (HDR), the CRISPeasy-in method (or crIN), referring to knock-in. This part was used especially for the second approach (CRIN).
Figure 3: CRISPeasy subdivisions.
2. CRIN (CRISPeasy-in method): Designed for knock-in strategy for bacterial genome editing based on CRISPR/Cas9
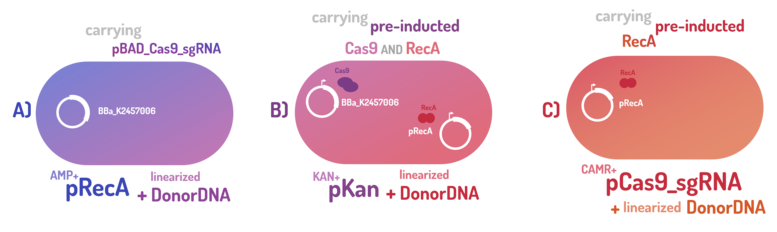
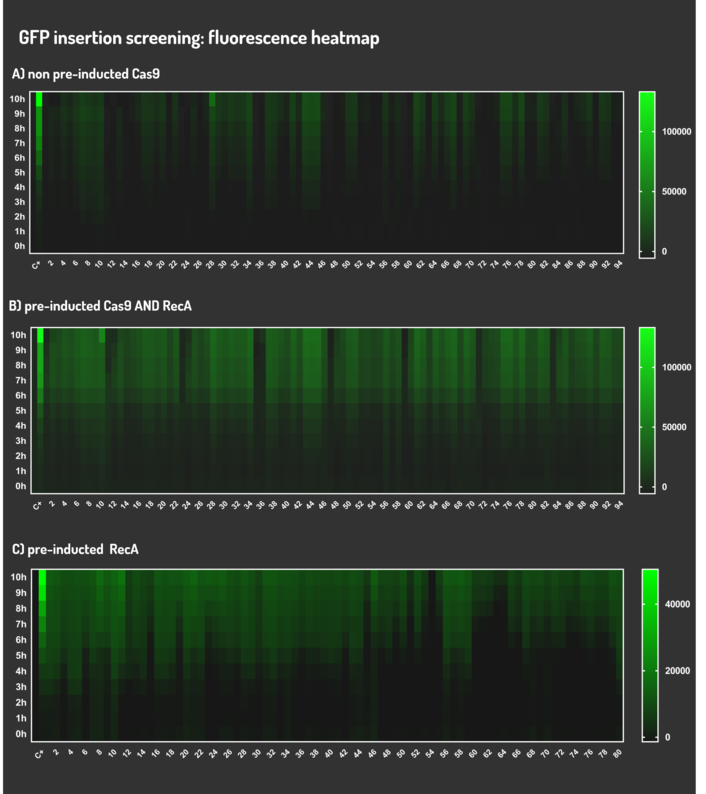
As the main key to this method, there is an AND logic gate composed of Cas9 coupled with RecA, which operates the Donor DNA insertion into E. coli genome through Direct Homology Repair (HDR). In this way, the arabinose AND IPTG (the inputs) activate the gene expression program encoding Cas9 and RecA. Then, there is the execution of their hardware's (physical parts, e.g. proteins) operation. As an output, Cas9 AND RecA activate the DNA insertion into the genome. In order to standardize a reliable datasheet of genome engineering efficiency, we tested a range of electrocompetent E. coli K12 MG1655 cells with different circuits: test A) carrying a non-pre-inducted Cas9 device + sgRNA (BBa_K2457006) plasmid; B) carrying a pre-inducted Cas9 AND RecA; C) carrying a pre-inducted RecA. The donor DNA was electroporated in all the tests.
Figure 4: CRISPeasy-in methodology.
A) carrying a non-pre-inducted Cas9 device + sgRNA;
We transformed through electroporation E. coli K12 MG1655 carrying a non-pre-inducted pBAD_Cas9_sgRNA plasmid CamR+ (BBa_K2457006) with pRecA (AmpR+) and a linearized Donor DNA (BBa_K2457004). We screened over 94 transformants colonies, inoculating in 200µL of LB + 25µg/mL chloramphenicol + 100µg/mL ampicillin + 0.2% L-Arabinose + 1mM IPTG to activate the expression of RecA AND Cas9. We measured the initial OD (600nm) and initial fluorescence (exc. 340 nm em. 530 nm) on 96-wells plates applying the Hidex Chameleon V spectrofluorimeter. The process was repeated for each hour over 10 hours while the 96-wells plate kept growing on a shaker at 37ºC.
B) carrying a pre-inducted Cas9 + sgRNA AND RecA;
We transformed through electroporation E. coli K12 MG1655 carrying a pre-inducted pBAD_Cas9_sgRNA plasmid CamR+ (BBa_K2457006) AND pRecA plasmid AmpR+ with a linearized Donor DNA (BBa_K2457004) + pKana. We screened over 94 transformants colonies, inoculating in 200µL of LB + 50µg/mL kanamycin + 25µg/mL chloramphenicol + 100µg/mL ampicillin + 0.2% L-Arabinose + 1mM IPTG to activate the expression of RecA AND Cas9. We measured the initial OD (600nm) and initial fluorescence (exc. 340 nm em. 530 nm) on 96-wells plates applying the Hidex Chameleon V spectrofluorimeter. The process was repeated for each hour over 10 hours while the 96-wells plate kept growing on a shaker at 37ºC.
C) carrying a pre-inducted RecA.
We transformed through electroporation E. coli K12 MG1655 carrying a pRecA (AmpR+) with pBAD_Cas9_sgRNA plasmid CamR+ (BBa_K2457006) and a linearized Donor DNA (BBa_K2457004). We screened over 80 transformants colonies, inoculating in 200µL of LB + 25µg/mL chloramphenicol + 100µg/mL ampicillin + 0.2% L-Arabinose + 1mM IPTG to activate the expression of RecA AND Cas9. We measured the initial OD (600nm) and initial fluorescence (exc. 340 nm em. 530 nm) on 96-wells plates applying the Hidex Chameleon V spectrofluorimeter. The process was repeated for each hour over 10 hours while the 96-wells plate kept growing on a shaker at 37ºC.
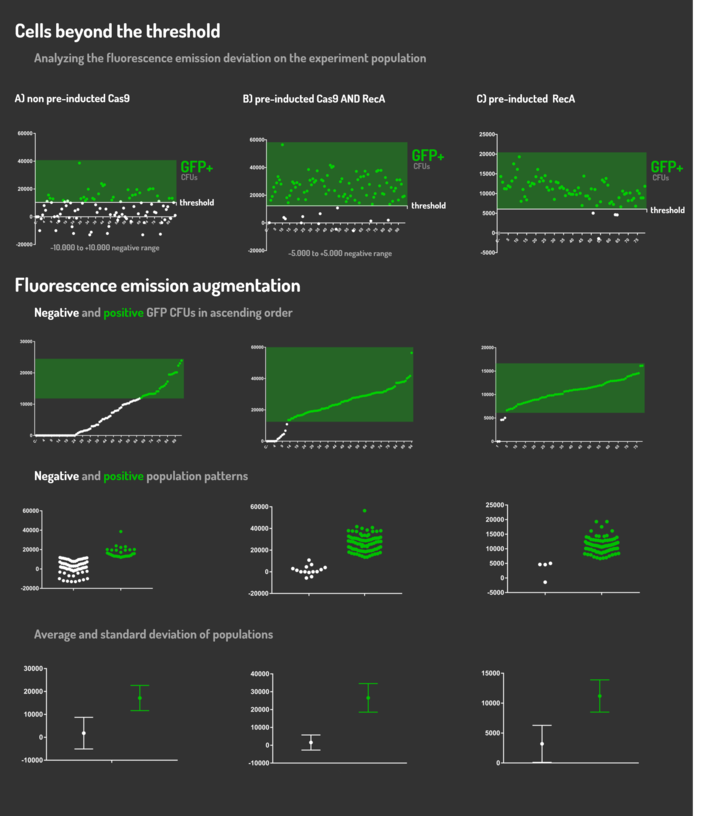
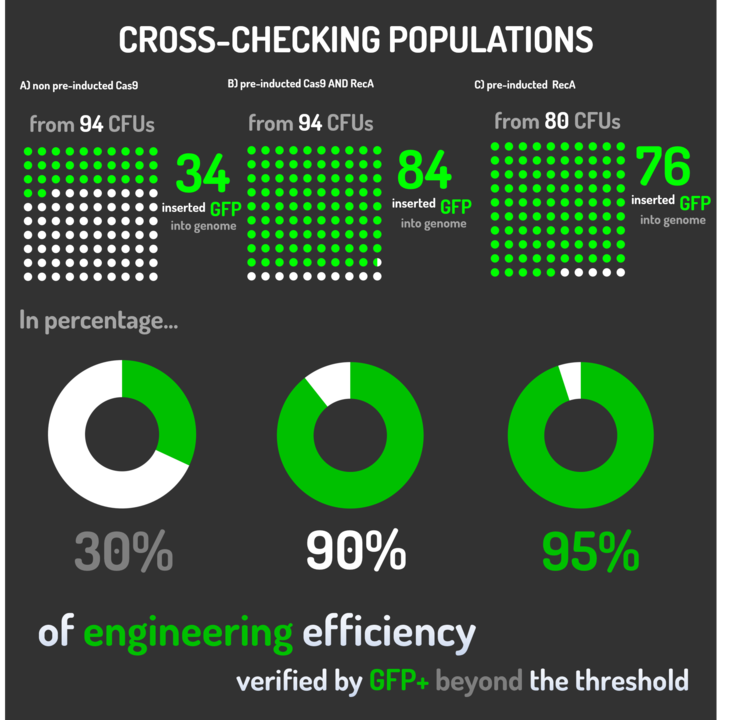
Figure 5: CRISPeasy-in results.
Leading from the achieved results, it was noted that has certain variations between the methodologies performances: with non-induced Cas9 (30% recombinants), with pre-induced Cas9 and RecA (90% recombinants) and with exclusively pre-induced RecA (95% recombinants).
The non-induced Cas9 method possibly do not have the best performance (30%) due to the absence of Cas9 protein when the Donor DNA is inserted into genome, causing a delay in the target sequence cleavage by Cas9, and, therefore, also the delay in homologous recombination, then the major linear Donor DNA is degraded in the bacterial cytosol.
The pre-induced Cas9 and RecA presented a very good result and similar to the most effective approach. When Donor DNA is inserted into the genome, is already happening the cleavage by Cas9 and RecA is investigating a homologous sequence to the broken strand, then Donor DNA is quickly incorporated, having less DNA degradation.
We found out that the most efficient method was the one which had only RecA pre-induced, since Cas9 presence affects the cells growth, therefore, when Cas9 and Donor DNA are added simultaneously, it will have the normal cell growth, beyond the further homologous recombination.
The CRISPeasy framework development can be a boost for an easily AND reliable way to use bacterial genome editing based on CRISPR/Cas9. We encourage teams to dive into CRISPR's standardization with us and expect to be able to see in the next years more and more projects with technique's improvement!
Sequencing
 Figure 6:Sequencing electropherogram from BBa_K2457003.
Figure 6:Sequencing electropherogram from BBa_K2457003.
Figure 7:Alignment of the designed sequence and our final construction from BBa_K2457003.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 508
Illegal AgeI site found at 1012 - 1000COMPATIBLE WITH RFC[1000]
| None |