Part:BBa_K2170214
Signal peptide BM40 (from basement membrane protein 40) in RFC[25] N-part
Signal peptide BM40
Results of signal peptide functional analysis
Bioinformatic signal peptide analysis via the SignalP server
The [http://www.cbs.dtu.dk/services/SignalP SignalP] server, being able to discriminate between the hydrophobic signal peptide sequence and the hydrophobic transmembrane domain[1], can determine whether a sequence possesses the biophysical requirements for functioning as a signal peptide. Furthermore, it is able to predict the probable cleavage site of the signal peptide after its translocation into the ER. For all constructs, signal peptide functionality is predicted, as well as a potential cleavage site. The complete translated amino acid sequences of the respective receptor constructs were used as input. The algorithm for eukaryotes with default D-cutoff value was chosen.

For all three signal peptides, the high S-score indicates high signal peptide functionality. For the Igκ signal peptide, a cleavage site between amino acid 20 and 21 within the signal peptide is predicted. For the BM40 signal peptide, a cleavage site between amino acid 17 and 18 is predicted, and for the EGFR signal peptide, a cleavage site between amino acid 24 and 25 is predicted.
Quantification of signal-peptide mediated translocation via a secretion luciferase assay
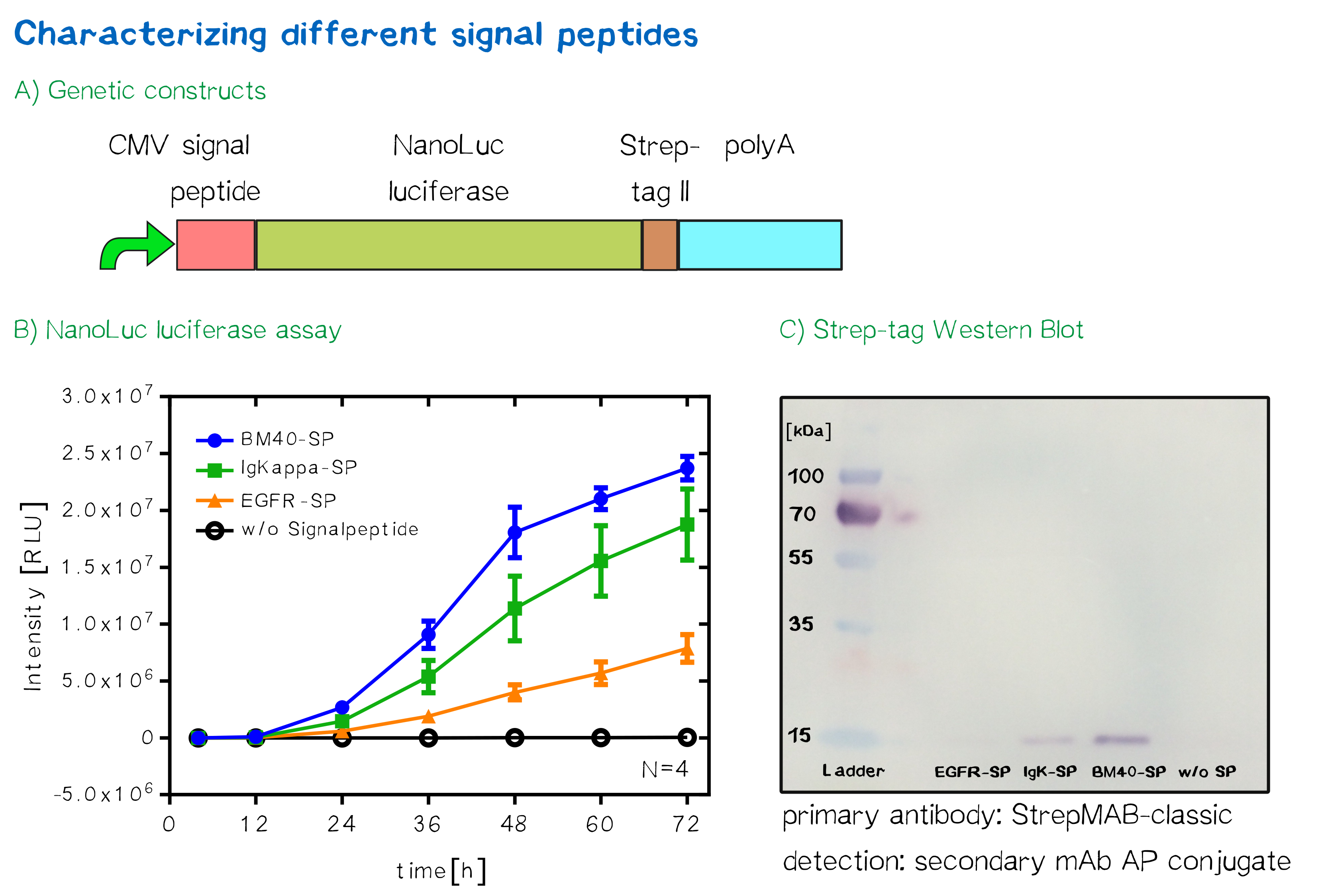
For the quantification of signal peptide functionality via a luciferase assay, three constructs were created and tested - each containing one of three signal peptides (the EGFR signal peptide, the Igκ signal peptide or the BM40 signal peptide) and a nanoluciferase as well as a CMV promoter, a Strep-tag II for immunochemical detection and the hGH polyadenylation signal sequence. Not containing a transmembrane domain, the nanoluciferase fusion protein is being translocated into the ER and then secreted into the medium. Using a luciferase assay, one can quantifiy the amount of luminescence - and thus, proportionally, the amount of secreted luciferase - by measuring the conversion of luciferin into visible light and integrating it over a timespan of 5 s. Therefore, medium samples were taken every 12 h after transfection of cells and measured via the Promega NanoGlo® luciferase assay system according to the manufacturer's instructions.
Discussion: The choice of signal peptide was nailed down
As would be expected, the EGFR signal peptide construct showed the lowest level of secretion. Although one might think this could also be attributed to a lower functionality of the signal peptide sequence itself after being translated, the lower secretion level is more likely to stem from lower translation initiation levels. Almost completely missing a 5’ UTR as well as a Kozak sequence, slower translation initiation (being a rate-limiting step) is likely to have lowered the level of translated protein in total. The constructs containing the Igκ and the BM40 signal peptide, on the other hand, contain a longer 5’ UTR as well as the Kozak consensus sequence, and thus result in higher secretion levels. Since the BM40 signal peptide hereby showed the highest secretion levels, it was thus incorporated into the receptor constructs.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
- ↑ Petersen, T. N., Brunak, S., von Heijne, G., & Nielsen, H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature methods, 8(10), 785-786.
| None |
