Part:BBa_K1893009
STAR inducible superfolder GFP (pAD1.S5+SF-GFP)
This is the STAR reporter plasmid containing the STAR target sequence upstream to the reporter gene SFGFP. Transcription of SFGFP is also controlled by constitutive promoter j23119 and terminator TrrnB.
Usage and Biology
When transcribed, the STAR target produces the sense RNA which is also complementary to the STAR-antisense RNA. In the absence of STAR, the STAR target DNA sequence is always transcribed and its sense-transcript forms the attenuator hairpin structure which facilitates RNAP drop-off and thus prevents transcription of the downstream reporter gene SFGFP. However when STAR-antisense is present, it binds to the the 5' stem of the sense STAR target sequence, preventing terminator hairpin formation and thus allowing transcription elongation of the downstream coding sequence.
Characterisation
We generated a two-plasmid system for characterisation experiments. The first plasmid contains the STAR sequence downstream of a constitutive Anderson promoter and followed by the t500 transcriptional terminator on a high-copy plasmid. The second plasmid is a reporter plasmid that contains the superfolder GFP (SFGFP) gene with a ribosome binding site immediately downstream of the STAR-target (pAD1 plasmid attenuator) sequence. The SFGFP coding sequence is under the control of a constitutive Anderson promoter and also has its own TrrnB terminator.
We characterised STAR activity at 30 and 37 degrees Celsius by using a plate reader to record the fluorescence of SFGFP over 200 minutes in cells with both plasmids, as well as in cells with just the reporter plasmid to determine to what degree STAR was able to activate SFGFP transcription.
Initially, we characterised the STAR system in terms of fold activation of GFP expression from the reporter plasmid in the absence and presence of STAR molecules. For this experiment, Top 10 E. coli cell lines were co-transformed with either the reporter plasmid and a plasmid with the J23119 promoter (no STAR) or with the reporter plasmid and the J23119-STAR plasmid. Cell cultures of these two cell lines were grown in microplates and well fluorescence was monitored over time using a microplate reader. The fluorescence signal from each well was normalised by dividing with the O.D. 600 value of that well. This gave the normalised fluorescence value for that cell line (FI/Abs). To account for cell autofluorescence, DH10B cells (similar to Top10) were used to determine background normalised fluorescence value.
Next, an experiment was carried out to investigate STAR system functionality at different temperatures. RNA elements functionality can be strongly depended on temperature and thermodynamics. In the case that we needed to use the STAR system under different circumstances (co-culture with B. subtilis that grows better at 30 degrees Celcius) we characterised the STAR system at 30 degrees Celsius cell culture condition. Cell cultures of the previously mentioned cell lines were grown for 5 hours in exponential phase. Then, the cell cultures were normalised at O.D. 600 = 0.4 and the fluorescent signal for each condition was recorded.
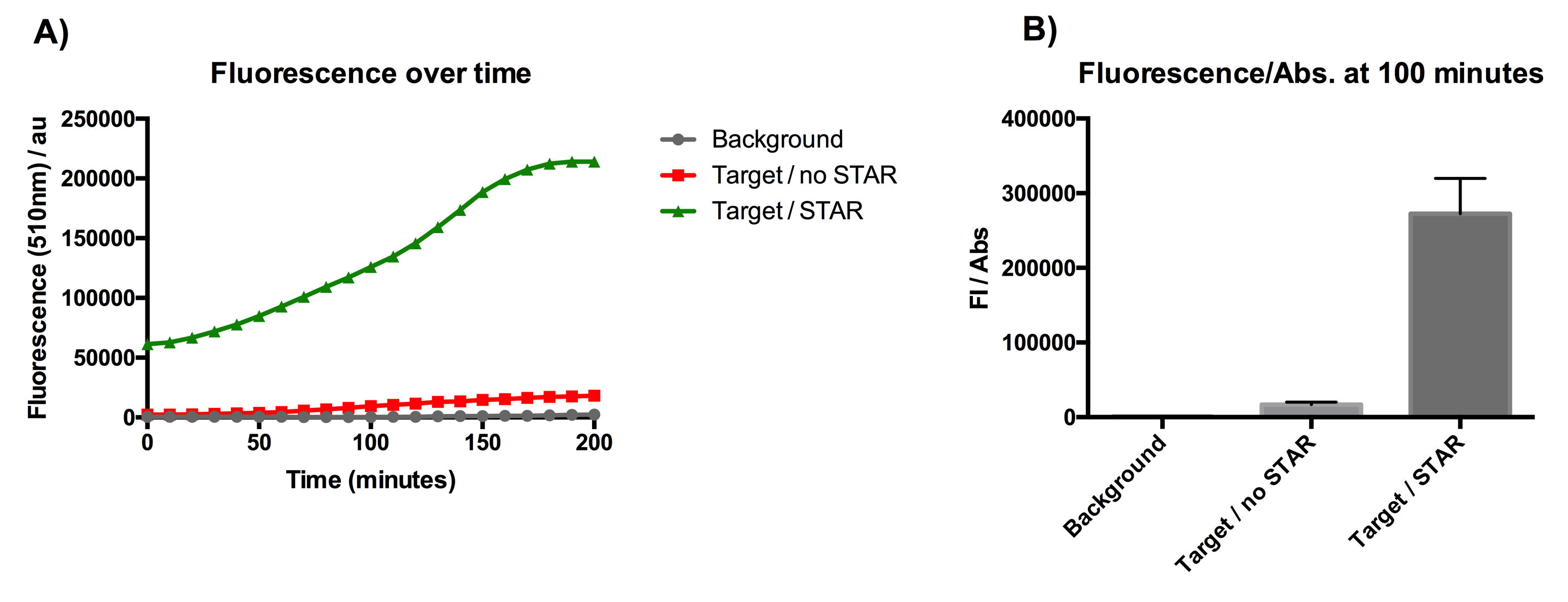
Figure 1: Characterisation of STAR system in TOP10 E. coli cells. (A) Normalised fluorescence monitored over time for cell lines incorporating the STAR system in the absence or presence of transcribed STAR molecules (B) Normalised endpoint fluorescence (100 minutes) for cell lines in the absence or presence of STAR molecules. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. Normalised fluorescence was calculated by dividing fluorescent signal by the O.D.600 value of the culture. Background was determined by the use of DH10B cells with no plasmid transformed. Error bars represent standard deviation from 3 technical repeats.
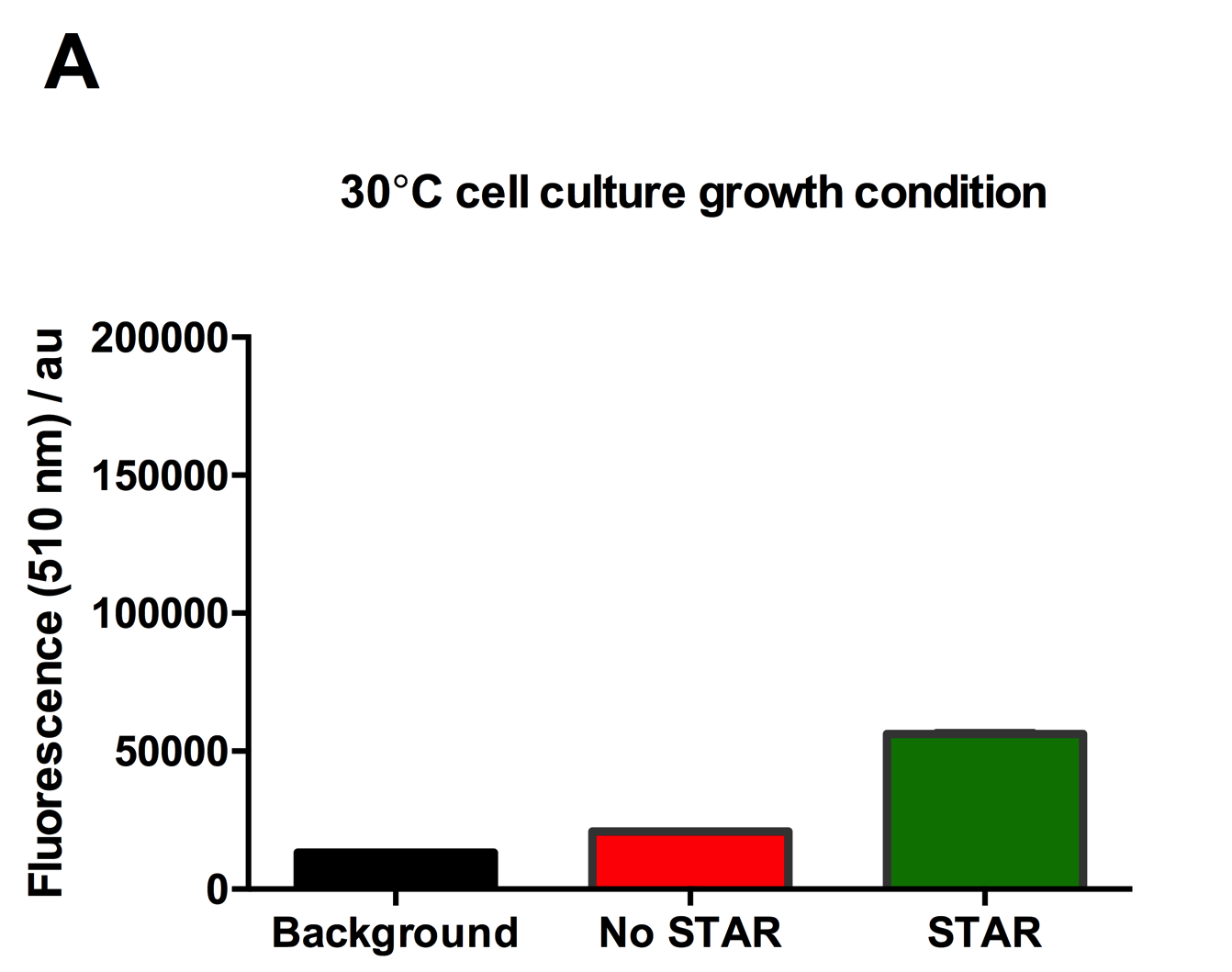
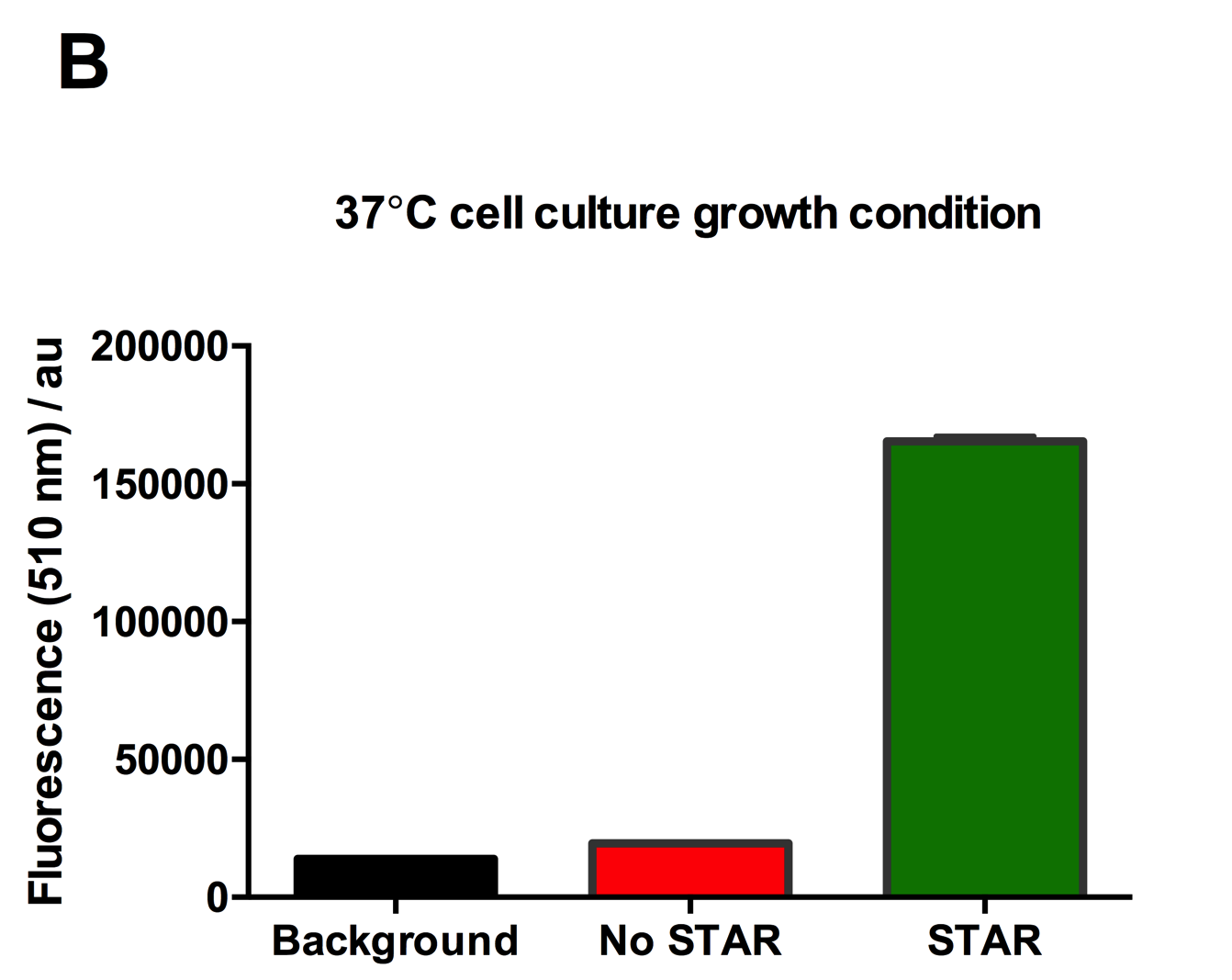
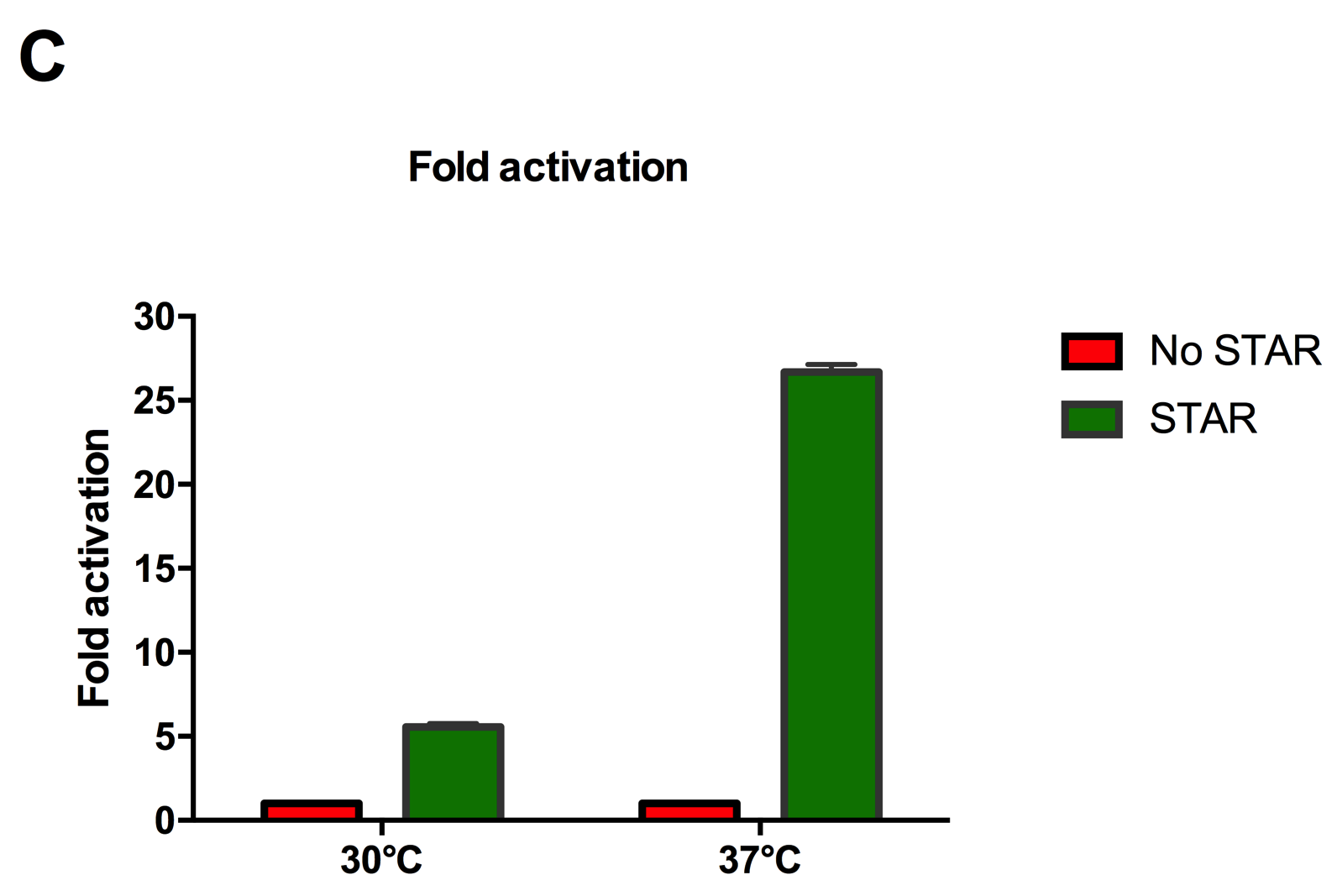
Figure 2: Characterisation of STAR system in TOP10 E. coli cells at different temperatures. (A) Cell culture fluorescence at 30°C (B) Cell culture fluorescence assay at 37°C. (C) Fold activation SFGFP expression in presence of STAR. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. The autofluorescence background control used is E. coli Top 10 cells with no reporter plasmid. Error bars represent standard deviation from 3 technical repeats.
Analysis
The results displayed in Figure 1A show that the expression of SFGFP is greatly increased in the presence of STAR over the course of 200 minutes of culturing. The graph from Figure 1B represents the normalised fluorescence once the growth data (optical density) has been taken into account. The data from this graph shows that SFGFP expression is increased more than 17-fold at 100 minutes of culturing, when STAR is present. This indicates that STAR is successful in preventing the pAD1 plasmid attenuator from interfering with SFGFP transcription. The data suggests that STAR is a promising tool for the regulation of the growth repressing gene in our circuit.
Experimental results showed that STAR system activated transcription at various levels at different temperatures. Figure 2A shows that SFGFP expression at 30°C increases significantly in the presence of STAR molecules as determined by the cell culture fluorescence. Figure 2B shows that SFGFP expression at 37°C increases significantly in the presence of STAR molecules as determined by the cell culture fluorescence, in agreement with our previous results. Figure 2C shows that the activation SFGFP expression attainable by the use of the STAR system was much greater at 37°C (26.7 fold) rather than at 30°C (5.5 fold). The results showcase important considerations that need to be taken into account when the STAR system is used under different culture conditions, and can be incorporated into the models of the system to inform design.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 569
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 959
Illegal BsaI site found at 1137
| None |