Part:BBa_K1732006
pSB1C3-J23100-B0034-Firefly-B0015
J23100-B0034-Firefly-B0015
J23100 Promoter (BBa_J23100) with RBS (BBa_B0034), Firefly Luciferase Sequence (BBa_I712019), and B0015 Terminator (BBa_B0015).
Firefly Luciferase, also known as FLuc, is a luciferase from Photinus pyralis. It is a protein that emits light when reacted with the appropriate substrate luciferin. It is able to act as a report protein when linked to other promoters of proteins. The luminescence generated can also be used to measure the luminescence of standard luminometers and compare it to the measurements by DIY luminometers [1]. This sequence was codon optimized to allow maximal amounts of proteins to be created and extracted.
Bioluminescent Mechanism of Luciferin
Firefly Luciferin is found in many Lampyridae species and serves as a substrate for luciferase enzymes. It is responsible for the yellow light emission of fireflies. For this study, Firefly Luciferase interacted with luciferin to determine relative light output and kinetics [3].
In the mechanism, luciferin incorporates ATP and magnesium ions as cofactors. In the presence of oxygen, AMP and carbon dioxide is released, resulting in the emission of a photon [2].
The image above is the bioluminescent mechanism of luciferin.
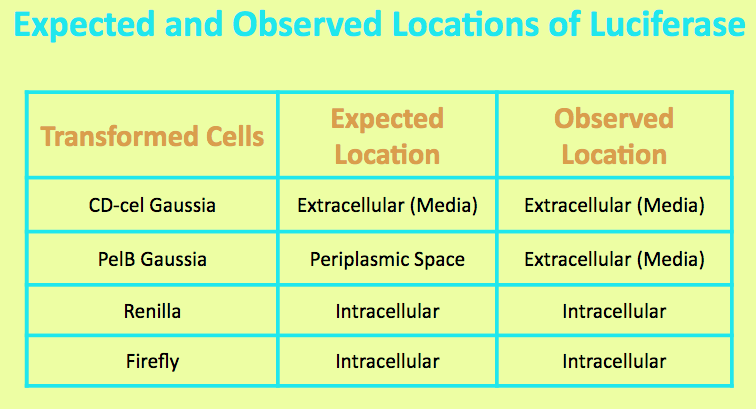
Before running experiments with Firefly Luciferase, the location of the luciferase in the media was determined by comparing the light output of overnight grown culture of Firefly cells with those of the pellet and the supernatant. The Firefly culture was spun down, and the pellet represented intracellular localization while the supernatant represented extracellular localization.
It was expected that Firefly Luciferase was localized in the cell, and the level of light output from the pellet matched that of the overnight culture and was significantly higher than that of the media. This suggested that the luciferase is located intracellularly.
The graph shows the amount of light output produced from varying concentrations of luciferin when added to 100uL of Firefly cells grown overnight (5mL LB broth, 5uL Chloramphenicol, single cell colony). 10uL of luciferin was added and this volume stayed consistent. The overnight culture was diluted 1/10 with LB broth in order to measure the Klett OD and stay within accurate measurement range. The purpose of this is to see the effect of light output with increasing concentrations of luciferin. The goal was to find a concentration that plateau without maxing out the luminometer (TECAN). The original stock solution was 30mM, so it cannot be determine at what concentration of luciferin saturates the sample.
The two types of competent cells used were Mach and Top10 cells. Mach cells are one of the fastest growing competent strain and Top10 cells also have transformation and cloning efficiency. Both are able to replicate high number of plasmids at stable levels.
From the graphs, it can be determined that higher concentrations of luciferin allows for a higher light output, but the decay rate is more significant . In addition, the plateau level steadies at a higher light output when given higher concentrations of luciferin. It is also important to note that the plateau occurs quickly and the jump in light output is temporary.
References
[1] [2]Gonzalez, V. M., Jr. (2007). Synthesis, Luminescence, and Applications of Coelenterazine and its
Analogs. University of Illinois Urbana-Champaign.
[3]Green A, McElroy WD (October 1956). "Function of adenosine triphosphate in the activation of luciferin". Arch. Biochem. Biophys.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 465
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 370
Illegal AgeI site found at 1640 - 1000COMPATIBLE WITH RFC[1000]
| None |