Part:BBa_K1692021
PanK + hybrid promoter phaCAB
Using pantothenate kinase (panK) from S. aureus to increase the production of poly-3-hydroxybuterate (P[3HB])
For our team's project, biOrigami, we wanted to increase the yield of P(3HB) that could be produced in vivo by E. coli.
P(3HB) is the most common polyhydroxyalkanoate, which is a group of fully biodegradable polymers with properties similar to those of petroleum-based plastics. It can be produced in a variety of microorganisms, and its synthesis as a compound for energy storage in vivo has been extensively studied [1].
Increasing the yield of P(3HB) from a cell culture would decrease the time needed to complete the plastic production and extraction process by making smaller cultures more efficient. We have used the phaCAB operon from Ralstonia eutropha H16 that encodes the three genes required for P(3HB) production: PhaA, PhaB, and PhaC. Previous iGEM teams (Tokyo Tech 2012 and Imperial College 2013) were able to successfully produce P(3HB) in vivo using BioBricks that encode these three genes. The pathway for P(3HB) production is as follows. First, 3-ketothiolase, encoded by PhaA, combines two molecules of acetyl-CoA to form acetoacetyl-CoA. This is then reduced by acetoacetyl-CoA reductase, encoded by PhaB, to form (R)-3-hydroxybutyl-CoA. This is then polymerized by PHA synthase, encoded by PhaC, forming poly-3-hydroxybuterate.
To increase the yield of P(3HB), we looked for a way to increase the amount of acetyl-coA, the precursor molecule to P(3HB) formation. Acetyl-CoA is composed of an acetyl group bound to coenzyme A. Coenzyme A consists of a beta-mercaptoethylamine group linked to pantothenic acid. On the Tokyo Tech 2012 iGEM team’s wiki, their results showed that adding pantothenic acid into their culture media led to increased yields of P(3HB). Therefore, we decided to increase the production of coenzyme A, which would then allow the cells to synthesize greater amounts of P(3HB).
Coenzyme A biosynthesis is a five-step process that is primarily regulated by the first enzyme in the pathway, pantothenate kinase (encoded by the gene PanK, which is also called CoaA) [2]. Pantothenate kinase stringently controls amount of coenzyme A produced in E. coli because the pantothenate kinase produced by E. coli is significantly inhibited by coenzyme A and its thioesters (such as acetyl-coA). Therefore, since we wanted to increase coenzyme A synthesis, we had to find a way to get around this negative feedback inhibition. Fortunately, the pantothenate kinase made in Staphylococcus aureus does not experience feedback inhibition from coenzyme A or its thioesters [3]. Indeed, this allows S. aureus to accumulate high levels of coenzyme A. We ordered the S. aureus pantothenate kinase sequence from IDT and inserted into the backbone PSB1C3 in front of the phaCAB operon designed by the Tokyo Tech iGEM team in 2012 with the hybrid promoter designed by the Imperial College iGEM team in 2013. The results section below shows that inserting the gene for pantothenate kinase allows for higher levels of P(3HB) production in E. coli.
This biobrick is a composite part of the panK gene (BBa_K1692020) before the part from the 2013 Imperial College team's part (BBa_K1149051).
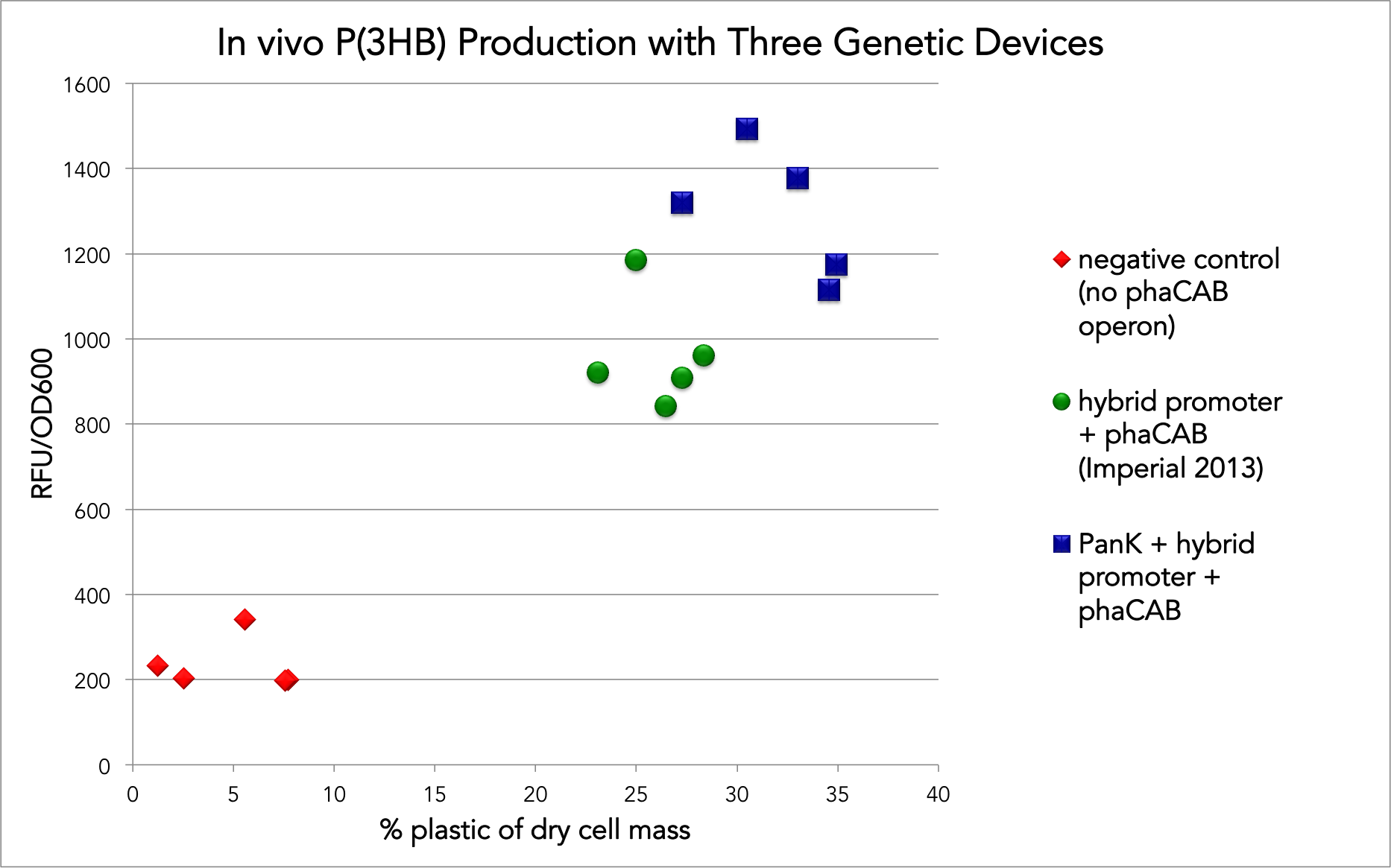
Our experiments focused on testing the benefits of adding the S. aureus panK gene in front of the Imperial College 2013 team’s device. We measured the amount of P(3HB) produced in vivo in two ways: by extracting the plastic and measuring its mass, and by staining the cultures and measuring the fluorescence on a flow cytometer and fluorometer. We contributed our results from our tests with their construct to the "Experience" page on that BioBrick.
To extract the plastic, we used sodium hypochlorite to dissolve the lyophilized cells, freeing the plastic. Measuring the mass of the culture post-lyophilization and measuring the mass of the plastic once extracted, purified, and fully dried allowed us to determine the percentages of the cells that were taken up by P(3HB) granules and compare between devices. The addition of panK to the existing P(3HB) device allowed for, on average, a 23% increase in the amount of plastic accumulated in vivo as a percentage of dry cell weight.

Inducible Lysis System
One of the main difficulty after making P(3HB) in the cells is the extraction process. P(3HB) granules accumulate within the cells, which is then extracted by two common methods: solvent extraction and chemical disruption. Solvent extraction is usually done with chloroform and the process can be laborious, required cells to be dried and also require the use of a hazardous chemical. The second method is the use of sodium hypochlorite to breakdown the cell walls and other cell components. The problem with sodium hypochlorite is that it can degrade the molecular weight of P(3HB) based on the time of incubation and concentration used [4]. Because of the difficulty with extraction, we look onto methods that can add an inducible lysis gene to the system. You can learn more about our inducible plastic lysis system here (BBa_K1692034).
References:
[1] Anderson, A. J. and Dawes, E. A. Occurrence, Metabolism, Metabolic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates. Microbiological Reviews 54 (4), 1990. 450 – 472.
[2] Leonardi, R. et al. Coenzyme A: Back in action. Progress in Lipid Research 44, 2005. 125 – 153.
[3] Leonardi, R. et al. A Pantothenate Kinase from Staphylococcus aureus Refractory to Feedback Regulation by Coenzyme A. The Journal of Biological Chemistry 280, 2005. 3312 – 3322.
[4] Madkour, M. et al. PHA Recovery from Biomass. Biomacromolecules 14, 2013, 2963-2972 Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 8
Illegal NheI site found at 31
Illegal NheI site found at 912
Illegal NheI site found at 935 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1884
Illegal BglII site found at 2709 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1190
Illegal NgoMIV site found at 1261
Illegal NgoMIV site found at 1861
Illegal NgoMIV site found at 2173
Illegal NgoMIV site found at 2452
Illegal NgoMIV site found at 3104
Illegal NgoMIV site found at 3126
Illegal AgeI site found at 418 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 4970
| None |