Part:BBa_K1666005
LsrR of LuxS/AI-2 signaling pathway in Salmonalla
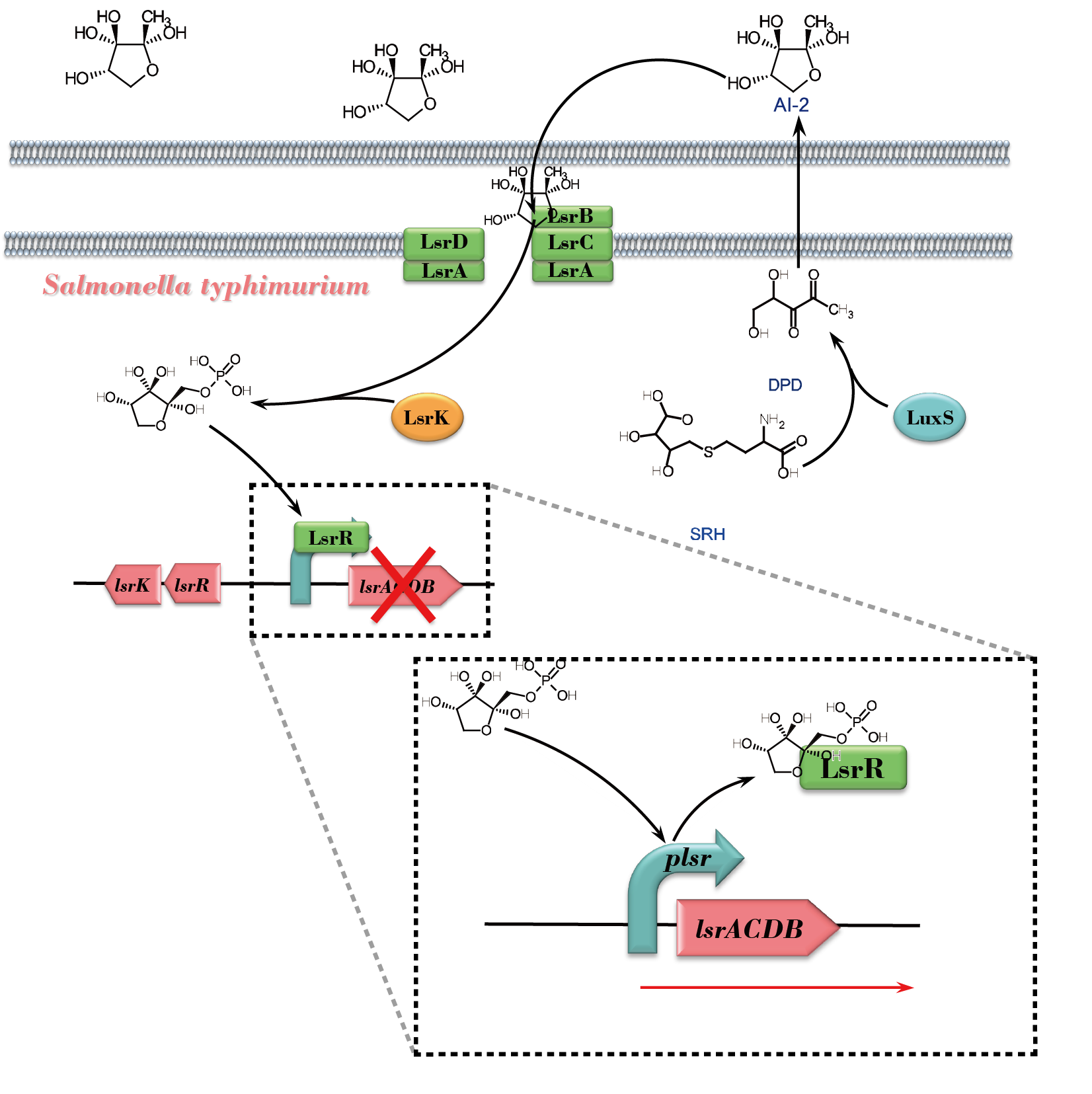
Quorum sensing is a process of bacterial cell-to-cell communication involving the production and detection of extracellular signaling molecules called autoinducers. And autoinducer-2 (AI-2) has been proposed to serve as a 'universal signal' for interspecies communication. In the LuxS/AI-2 signaling system of Salmonella Typhimurium, AI-2 response involves ATP binding cassette transporter encoded by genes named Lsr (LuxS regulated). And LsrR is the Transcriptional regulator which is inactivated by binding phospho-AI-2, leading to the transcription of the lsr genes. In our project, we set this protein-coding part under a nisA promoter and try to integrate them in the genome of Lactobacillus or Lactococcus for the final purpose of constructing an integrated AI-2 response pathway of Salmonella in the engineered bacteria.
Usage and Biology
AI-2 is generated by many species of Gram-negative and Gram-positive bacteria. In a group of bacteria exemplified by Salmonella, AI-2 response involves lsr genes that encode ATP binding cassette-type transporter. In the absence of autoinducer 2 (AI-2), LsrR represses transcription of the lsr operon and itself. Phospho-AI-2 can bind LsrR and inactivate it through releasing it from the repressed promoters, leading to the transcription of the lsr genes.
In our project, we set this protein-coding part under the regulation of a nisA promoter which can be activated by food-grade inducer, nisin. We linearized the related expression vectors and stably integrated them into the genome of the hosts. And together with other parts, we will construct a response pathway for AI-2 generated by pathogens in the engineered bacteria.
Characterization
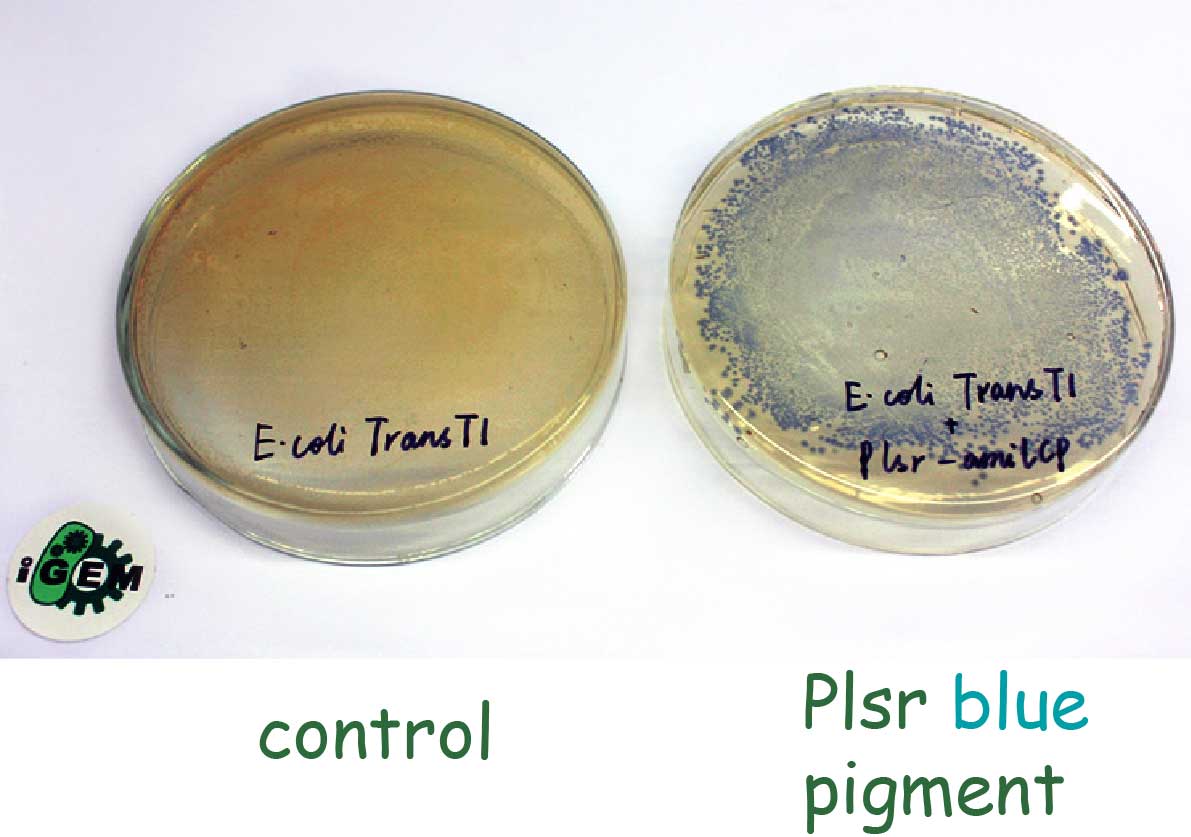
We transformed the plasmid pHY300PLK containing Plsr with a blue pigment gene at its downstream into E. coli Trans T1. As we can see, after overnight incubation, we observed blue colonies on the plate. That means without LsrR-mediated repression, the Plsr promoter will be constitutively active and promote the production of visible blue pigment.
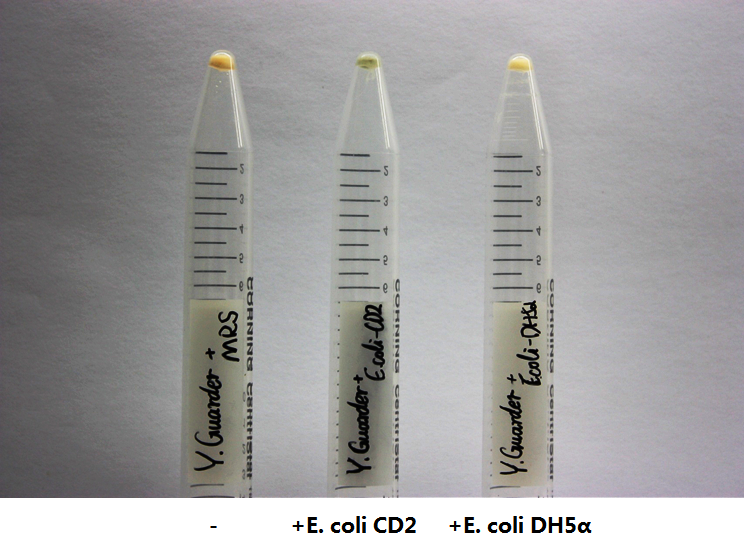
After we have successfully integrated pNZ9530 and the expression vectors for lsrB, R and K into the Lactobacillus genome, we sequentially transformed the Plsr-amilCP vector. We cultured our engineered Lactobacillus in the medium containing AI-2 secreted by E. coli CD-2. We used DH5alpha bacteria as a control because they do not produce any AI-2. Our engineered Lactobacillus showed clear blue color compared to the control. Without AI-2, the engineered bacteria did not generate blue pigment due to the repression of LsrR. The result strongly indicated that LsrR functions as expected.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 267
Illegal NgoMIV site found at 844 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 43
| None |