Part:BBa_K1618022
GlgB with IPTG-inducible promoter
GlgB cleaves alpha-1,4 linkages in glycogen chains and transfers the cleaved branch onto the main glycogen chain using an alpha-1,6 linkage to make a branch point. GlgB is preceded by the LacI IPTG-inducible promoter an an RBS.
iGEM15_NRP-UEA used this part in E. coli. Cells were transformed and grown10 mL LB media overnight at 37 °C with shaking. Each culture was used to inoculate 2 x 10 mL of fresh media, grown to an OD of approximately 0.6 and then IPTG was added to one of each duplicate culture and the cultures continued to grow, with samples taken after 1 hour, 3 hours and overnight. Glycogen was extracted from the cell pellet according to the following glycogen extraction protocol:
(1) Harvest E. coli cells from liquid cultures by centrifugation at 5000 rpm for 10 minutes and resuspended in 10 mL water in a 50 mL Falcon tube. (2) Pellet resuspended cells by spinning at 5,000 × g for 10 minutes. Discard supernatant and resuspend pellet in 10 mL fresh water. (3) Sonicate at room temperature at 10 micron amplitude for 3 minutes, 1 second on and 2 seconds off. (4) Transfer to 50 mL centrifuge tubes and centrifuge at 30,000 × g for 15 minutes. (5) Transfer supernatant to a 50 mL Falcon tube. Add 5 mL of 0.2 M glycine, pH 10.5 and 5 mL chloroform. Shake vigorously and spin at 2000 rpm for 3 minutes to separate into aqueous and organic layers. (6) Transfer top, aqueous layer to a new 50 mL Falcon tube with a pipette and repeat step 5. (7) Transfer top, aqueous layer to a round-bottomed flask and remove any remaining chloroform using rotary evaporation. (8) Transfer to a 30 kDa spin filter and concentrate to ~8 mL by spinning at 5000 × g for approximately 55 minutes. Check after 30 minutes of centrifugation. (9) Transfer to 8 × 1 mL ultracentrifuge tubes and balance all to within 1 mg of each other. (10) Spin in Ultracentrifuge at 108,000 × g (55,000 rpm) at 4ºC for 2-3 hours. (11) Discard supernatant and resuspend pellets in 2 mL total volume of water and add to 50 mL Falcon tube.v (12) Precipitate glycogen with 8 mL cold ethanol. (13) Spin at 4,000 × g for 10 minutes and discard supernatant. (14) Dissolve pellet in 2 mL of water and freeze-dry overnight to yield the glycogen as an amorphous white powder.
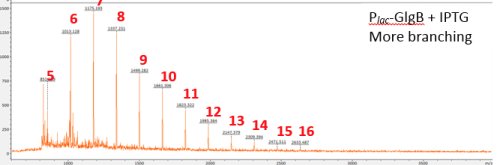
The extracted glycogen was dissolved in 100 uL 100 mM sodium acetate buffer, pH 4 and then treated with Pseudomonas Isoamylase (1 Unit) at 37 °C for 3 hours. Isoamylase removes α-1,6 linkages and de-branches the glycogen sample. A control sample of commercial glycogen was also debranched by this method. An aliquot of the glycogen was then diluted 100 fold in matrix solution (1mg/mL Dihydroxybenzoic acid in 30% aq. Acetonitrile) and analysed by MALDI mass spectrometry. When GlgB is overexpressed GlgB is overexpressed there is a higher proportion of shorter chain lengths and a lower maximum chain length can be observed:
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 283
Illegal BamHI site found at 1491 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 917
| None |