Part:BBa_K1149051:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1149051
User Reviews
UNIQ493e97d31a043af3-partinfo-00000000-QINU
|
•••••
Edinburgh OG 2018 |
E. coli cells were cultured with different concentrations of glucose at 37 ℃ and optical density was measured at cultivation time of 6 hours, 24 hours, 30 hours 48 hours, 56 hours and 72 hours.
Figure 1 Comparison of growth curve of recombinant E. coli harbouring pSB1C3-phaCAB for different concentration of glucose. The iGEM Edinburgh OG 2018 team decided to assess the Real-time PHA detection by Semi-quantitative analysis (Nile Red Fluorescent intensity-through using Image J program) Cell cultures were harvested at different cultivation hours including 6 hours, 24 hours, 30 hours and 48 hours. The bars in blue and orange represented signal intensity of cells harboured pSB1C3-phaCAB and pSB1C3 respectively. The bars in yellow represented signal intensity resulting from PHA, which were calculated from the difference between intensity of pSB1C3-phaCAB and intensity of pSB1C3.
Figure 2. Fluorescent intensity of cells harboured pSB1C3 or pSB1C3-phaCAB at different cultivation time.
The team then decided To determine the influence of glucose availability on PHA production level, in addition to the growth curves with different glucose concentrations.
Figure 3. The fluorescent intensity of PHA produced with different glucose concentrations. (48 hours cultivation). Error bars represented standard deviations.
Table 1. Yield of PHA of pSB1C3-phaCAB with different glucose concentrations Effect of different concentration of propionic acid. In order to determine the tolerance of higher concentration of propionic acid, E. coli harbouring pSB1C3-phaCAB plasmids were cultured with 3 % glucose and different concentration of propionic acid for 60 hours.
Figure 4. Comparison of growth curves with different concentration of propionic acid. E. coli harbouring pSB1C3-phaCAB was cultivated with different glucose and propionic acid. The time of adding propionic acid was pointed out by red arrow.
|
|
BBa_K1149051 4 Not understood victorpabst |
The iGEM Paris Bettencourt 2017 team decided to characterize the biobrick BBa_K1149051 using flow cytometry. By staining our cells with a Nile Red solution (0.3mg/mL of DMSO), a compound that becomes fluorescent when P3HB is present, we were able to quantify the amount of P3HB produced at the single cell level. The following protocol was used for staining
 Figure 1: Flow cytometer analysis of cell stained with NileRed with BBa_K1149051 Figure 1: Flow cytometer analysis of cell stained with NileRed with BBa_K1149051
A Beckman Coulter Life Science ® flow cytometer machine to measure fluorescence at the single cell level. For each of our 2 clones, 3 replicates were performed and a negative control, stained cells that do not carry the Pha operon. We took 1 µL of every sample including a negative control (from an overnight culture of wild type E.Coli DH5 α), and analyse them through the FL2 (575 BP filter) and FL3 (620 BP filter) channel. As shown on figure 1, there is clear production of P3HB from the cells when transformed with the biobrick containing the operon. The two biological replicates have a similar behavior and have both been shown to be significantly different from the negative control (ttest, p<0.0001 in both cases). We chose to work with flow Cytometry was used to further characterize the part because it allows for very accurate measurements and the study of a large amount of cells. This technique allows for the measurement of hundreds of samples each day at a minimal cost, whereas using GC/MS is not only expensive, but only a few samples can be run each day. |
UNIQ493e97d31a043af3-partinfo-00000004-QINU
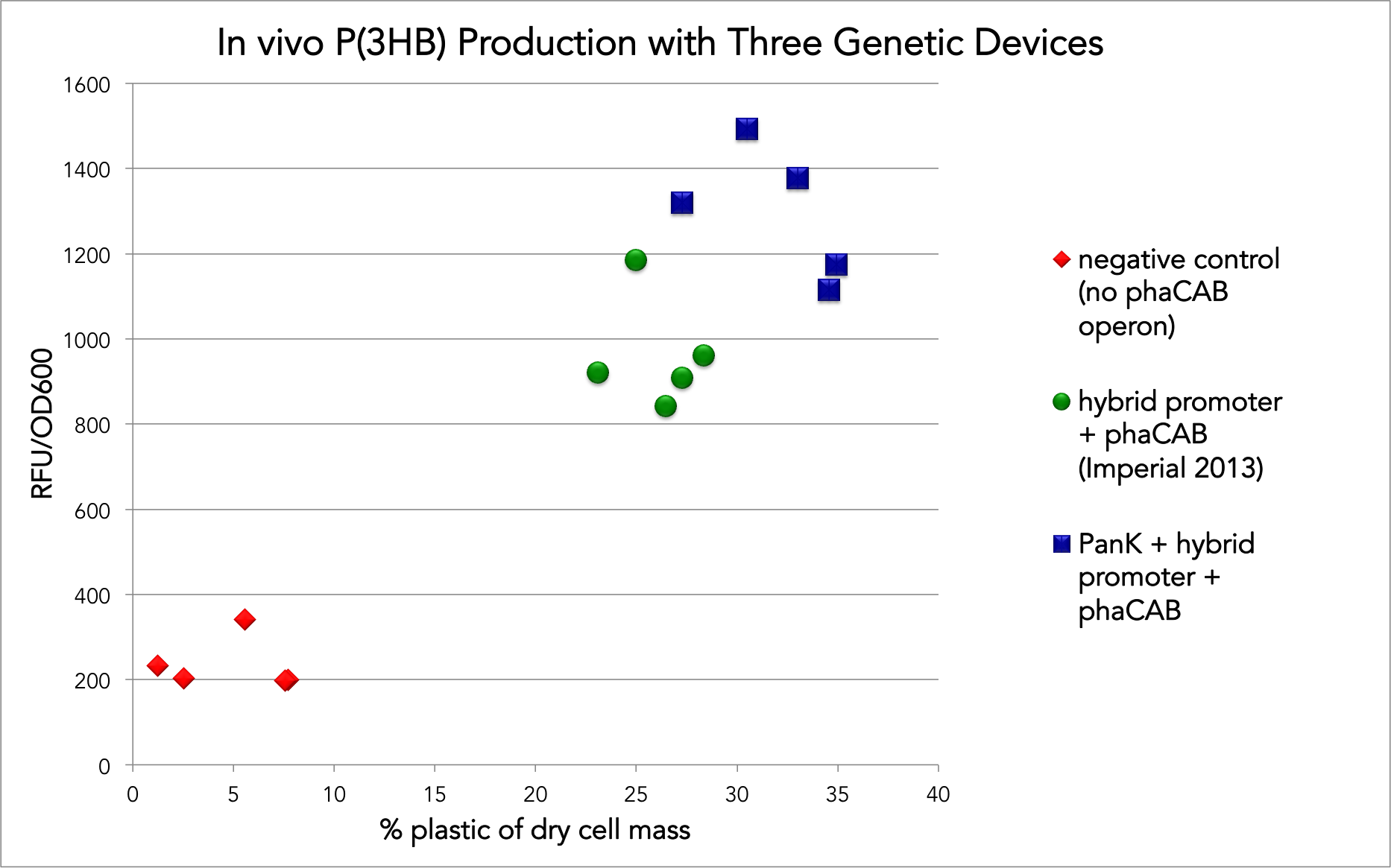
The Stanford-Brown 2015 team wanted to build off of the Imperial College team's work by adding the gene for a type II pantothenate kinase (panK) to this part to make a composite part (BBa_K1692021). To test whether the panK gene caused more plastic to be produced, we used this BioBrick as a comparison. Our final results did show that the addition of the panK gene did cause an increase in plastic production, as shown by the figure below.
Notably, we used the strain NEB5-alpha instead of the same strain as the Imperial 2013 team used (MG1655). NEB5-alpha is not known to be a high accumulator of P(3HB), but is is a common strain so we wanted to use it to test our construct. Even though our construct produced, on average, a 23% increase in the amount of plastic in vivo as a percentage of dry cell weight over the Imperial 2013 construct in our lab, we still produced less plastic than the Imperial 2013 did. As opposed to their value of 58.9% plastic, we were only able to achieve 25-34% yield. We suspect that is due to the strain difference, since the media difference should have worked in our favor (we used TB, which the Tokyo Tech iGEM 2012 team had shown causes more plastic to be produced). However, supplementing the media with glucose, as opposed to as with glycerol, could have also caused the difference in accumulation.
Calgary 2017: HPLC of PHB digestion in sulfuric acid
In order to further characterize the part, we carried out digestion of PHB produced from this part and analyzed the amount of crotonic acid produced. The protocol used for digestion is given on our page. The digestion times used were 20 mins (Low) and 30 mins (High). About 0.0249 g of PHB obtained from extraction was used for the experiment. The following figures show the HPLC results obtained.
Low

Figure 1. HPLC results from digestion of PHB in sulphuric acid for 20 mins.
High

Figure 2. HPLC results from digestion of PHB in sulphuric acid for 30 mins.
A standard curve was generated using PHB from Polyferm with known concentration of PHB. The concentrations of PHB used for standard curve were 0.01 mM, 0.25 mM, 0.5 mM, and 0.75 mM. The area of crotonic acid from HPLC results was then used to calculate the concentration of PHB in sample. The standard curve generated is shown below:

Figure 3. Standard curve to estimate concentration of crotonic acid in sample using Polyferm's PHB.

Table 1. PHB amount in mg calculated from amount of crotonic acid recorded in HPLC of samples digested for 20 mins (low) and 30 mins (high). The area of crotonic acid recorded in HPLC, dilution factor, and conversion factor were used to calculate the amount of PHB in the three samples
The HPLC results showed that crotonic acid was present in the samples with 20 mins and 30 mins of digestion time. Thus, as seen in literature that the presence of crotonic acid is a positive test for confirmation of PHB in sample.