Part:BBa_K1074000
TD1, Transdermal peptide
TD1 is a short synthetic peptide(ACSSSPSKHCG)identified by in vivo phage display, facilitated efficient transdermal protein delivery through intact skin. Studies suggested that the peptide creates a transient opening in the skin barrier to enable macromolecular material to reach systemic circulation.
As the largest organ of the human body, skin provides a painless interface for systemic drug delivery. However, the permeability of foreign molecules, especially large hydrophilic molecules, across the stratum corneum in the outer skin surface is extremely low.In the last 50 years a large number of chemical penetration enhancers(CPEs), belonging to several different classes such as surfactants,fatty acids, fatty esters and Azone-like compounds, have enabledlimited skin permeation enhancement. But CPEs have the limitations to deliver large molecules,especially large hydrophilic proteins. Now, TD1 has fulfilled this formidable task.
Small, efficient, easy implementation etc, TD1 offer a powerful experimental methodology for the transdermal protein delivery . And its nature of polypeptide make it an undoubtedly nice biobrick in synthetic biology . In our project,we fuse TD1 with a varity of antigens(HBsAg(Hepatitis B virus),Ag85b(Mycobacterium tuberculosis),PAD4(Bacillus Anthracis)) and adjuvant so they can be delievered through the skin to provoke the immune response to achieve the goal of "Transdermal vaccine".
Usage and Biology
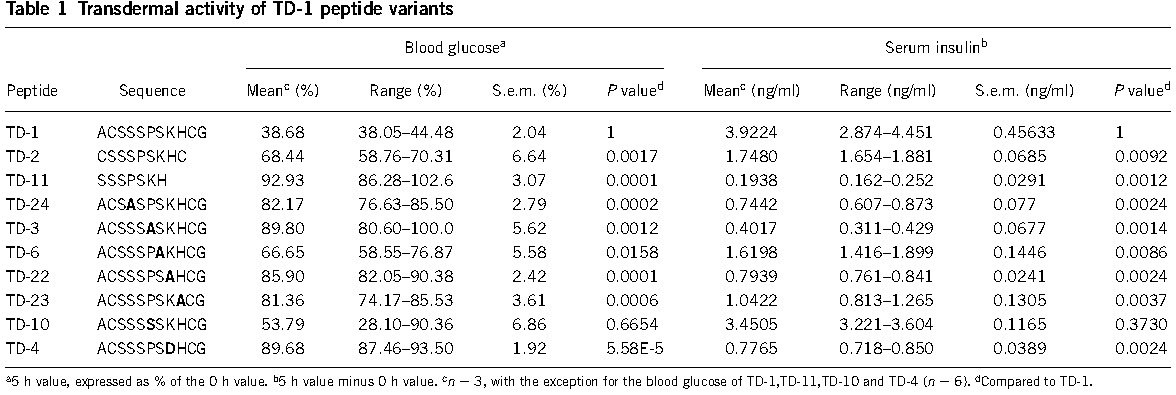
TD1 can facilitated transdermal protein delivery by Coadministration of it with target protein or fused with the target protein(always N-terminal).Studies shows that Coadministration of the peptide and insulin to the abdominal skin of diabetic rats resulted in elevated systemic levels of insulin and suppressed serum glucose levels for at least 11 h. Significant systemic bioavailability of human growth hormone was also achieved when topically coadministered with the peptide(figure 1).

The transdermal-enhancing activity of the peptide was sequence specific(figure 2) and dose dependent, did not involve direct interaction with target proteins(figure 3).

When you fuse TD1 with your target proteins, N-terminal modified and a linker of GGGS are recommended.
References
Chen, Y.P., et al., Transdermal protein delivery by a coadministered peptide identified via phage display. Nature biotechnology, 2006. 24(4): p. 455-460.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Further Characterization - UofC_Calgary 2016
The UofC_Calgary 2016 team used TD1 fused to the N-terminus of the modified version of the Bowman-Birk Protease Inhibitor (BBI), a small radioprotective peptide 14 amino acids in length (https://parts.igem.org/Part:BBa_K2008005). We performed in vivo trials in mice to determine if our peptide fused to the TD1 tag could pass from our delivery system, an adhesive patch, through the skin of the mice and into the blood. Patches were adhered to mice for a period of five days before the mice were euthanized and blood samples were taken. Mass spectrometry was used to analyze blood and serum samples to detect the presence of TD1-BBI.
Methods
1) Peptides were extracted from mouse blood plasma or serum via purification on a C18 Zip-Tip column from Thermo; eluted with 50% acetonitrile, 0.1% formic acid
2) Samples were concentrated 10x via evaporation and resuspended in 0.1% formic acid
3) Extracted samples were injected on to a 10 cm C18 column and eluted with a 30 min, 5-40%B gradient at 300 nL/min
4) MS spectra were acquired on a Thermo LTQ Orbitrap Velos in ion trap mode; MS/MS spectra were acquired in Orbitrap mode with CID
5) Spectra were manually analyzed for the presence of BBI (monoisotopic mass = 2577.1 Da) at various charge states; pure sample of BBI, synthesized by BioBasic, was used as a reference for identification
We acquired mass spectra for a synthetic BBI standard [tagged with TD1 and KSCI]. The characterization of a BBI standard will be of value to future experiments when detection of BBI is necessary. MS spectrum of intact BBI in multiple charge states is shown in Figure 4; fragmentation of the 3+ and 4+ charge states, via CID, is shown in figures 5 and 6, respectively.

Fig 4. Standard mass spectra with states denoted.

Fig 5. Mass spectra of BBI in a +3 state

Fig 6. Mass spectra of BBI in a +4 state
From these results, it was shown that TD1-BBI was not present in our mouse blood sample. This may indicate that TD1 does not facilitate the ease of transdermal transport, or that our TD1-BBI peptide was degraded in mouse blood before it could be. Further studies for characterization are required.
| None |