Difference between revisions of "Part:BBa K907000"
| Line 12: | Line 12: | ||
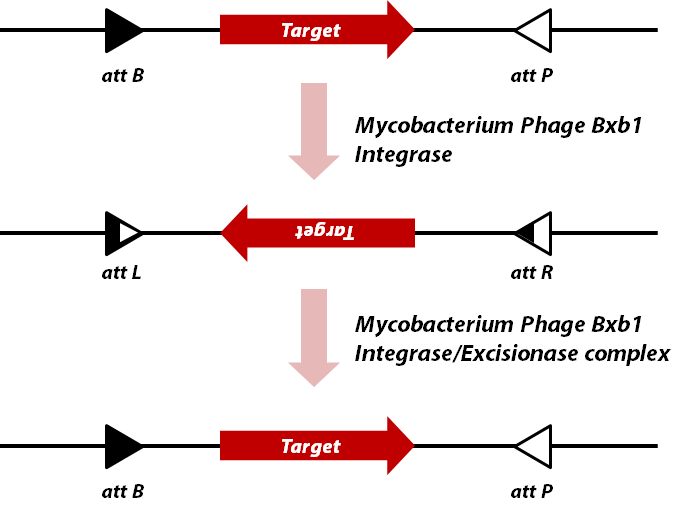
complex and this complex flips inverted DNA back into original sequence by regenerating ''attB'' and ''attP'' sequences. | complex and this complex flips inverted DNA back into original sequence by regenerating ''attB'' and ''attP'' sequences. | ||
---- ''' | ---- ''' | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | ||
| − | + | <ul> | |
<li>'''Group:''' ETH Zurich 2016 | <li>'''Group:''' ETH Zurich 2016 | ||
<li>'''Author:''' Asli Azizoglu | <li>'''Author:''' Asli Azizoglu | ||
<li>'''Summary:''' We cloned and characterised a codon optimised bxb1 for <i>E.coli</i>, and sent it to the registry as | <li>'''Summary:''' We cloned and characterised a codon optimised bxb1 for <i>E.coli</i>, and sent it to the registry as | ||
| − | a biobrick. Our biobrick can be found here with a fast degradation tag | + | a biobrick. Our biobrick can be found here with a fast degradation tag and here without any degradation |
| − | tags[[Part:BBa_K2116026]]. | + | tags[[Part:BBa_K2116026]]. The non-codon optimised version can be found here [[Part:BBa_K907000]] |
</ul> | </ul> | ||
Revision as of 20:26, 22 October 2016
Mycobacterium Phage Bxb1 gp35, DNA integrase
This part is a protein coding part which encodes
Bxb1 gp35, which is a DNA integrase of Mycobacterium phage Bxb1.
The Bxb1 integrase is a DNA recombinase, more precisely a member of serine integrase family. It recognizes specific sequences,
called attB and attP, and then integrates, inverts, or excises dsDNA depending on the orientation of recognition
sequences. We used this integrase to invert specific sequence in plasmid. The protein is well expressed in E.coli(strain
MG1655) When it inverts DNA sequence, the attB and attP sequences are changed into attL and attR, as other
DNA recombinases do. Another protein called Bxb1 gp47(BBa_K0907002) binds to integrase-DNA
complex and this complex flips inverted DNA back into original sequence by regenerating attB and attP sequences.
- Group: ETH Zurich 2016
- Author: Asli Azizoglu
- Summary: We cloned and characterised a codon optimised bxb1 for E.coli, and sent it to the registry as a biobrick. Our biobrick can be found here with a fast degradation tag and here without any degradation tagsPart:BBa_K2116026. The non-codon optimised version can be found here Part:BBa_K907000
Kinetic Characterisation
We investigated the kinetics of bxb1 flipping using flow cytometry.
Genetic Design
- Bxb1 was expressed under a tet promoter, without any degradation tags. The RBS has a strenght of 1209.69 au (translation rate), as calculated by the Salis RBS Calculator. This construct was cloned on a medium copy plasmid with the p15A replication of origin.
- TetR was expressed under the control of medium-strength constitutive promoter Part:Bba_J23118, and cloned onto a low copy plasmid with pSC101/Rep101 replication of origin.
- We also constructed a dual fluorescence reporter system Part:BBa_K2116024 that expresses GFP when bxb1 flips a directional promoter.
Experimental Setup
- The test construct with bxb1 was transformed together with the reporter construct, and either with TetR or with the empty backbone as control.
- Cells were grown in LB medium for 4 hours, and then transfered to minimal M9 medium with a 1:100 dilution. aTc was added at given concentrations at OD600. Samples were taken at the specified time points, spun down and resuspended in PBS at OD600 0.01 and kept on ice until measurement.
- Results are presented as mean fluorescence per cell and the standard error of the mean.
Results
We observed that maximum flipping is reached at around 8h under these conditions. The response correlates with aTc concentration. However compared to the control, where there is no TetR, we see 6-fold less flipping. This could be improved by increasing the concentration of aTc, however we have found that at 8000 ng/μL cell growth was inhibited.
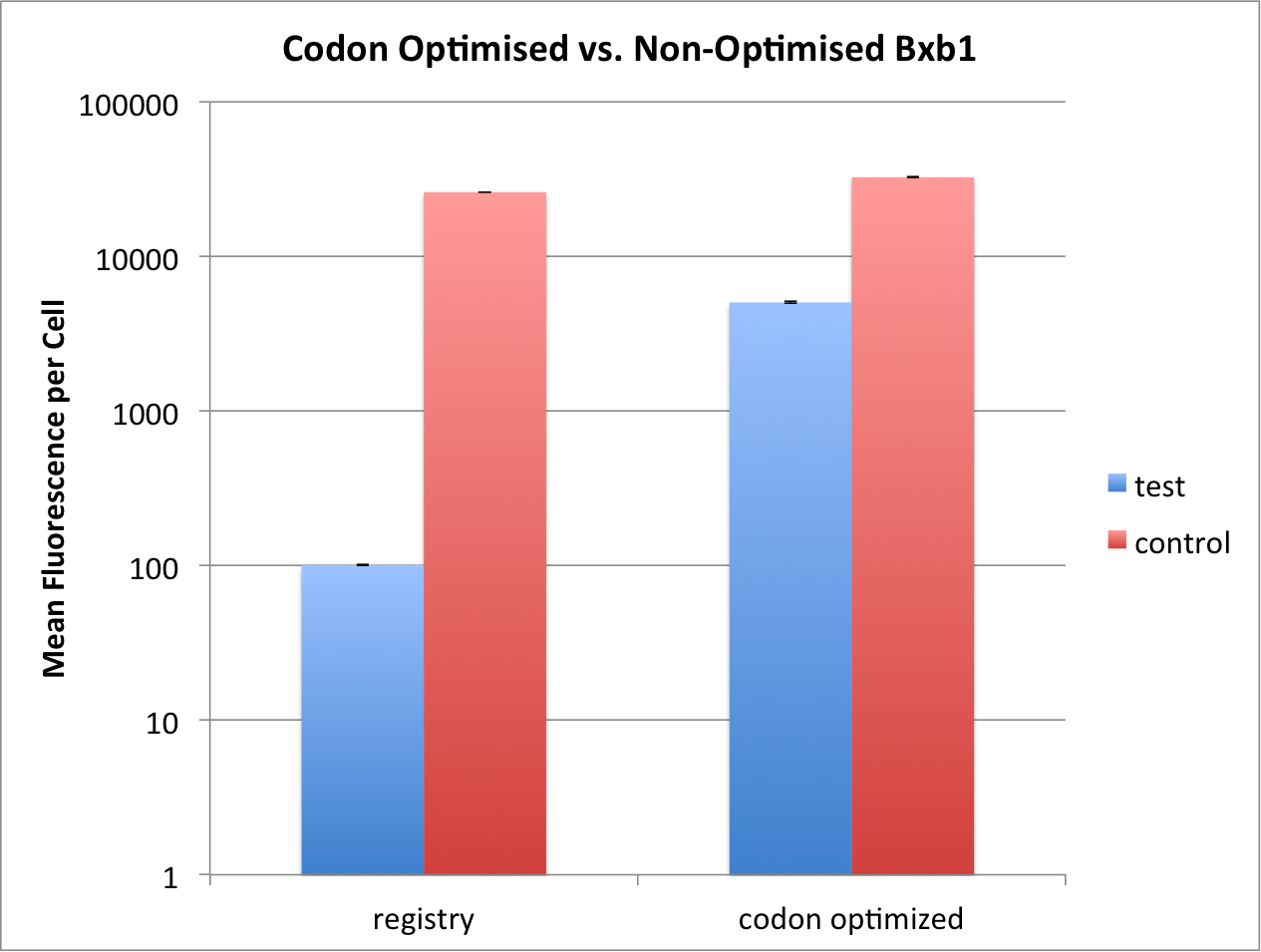
Comparison between Codon Optimised and Registry Bxb1 Integrase
We compared the kinetics of our codon optimised bxb1 Part:BBa_K2116026 and the one found previously on the registry Part:BBa_K907000 that was not codon optimised. The same genetic and experimental setup was used as above. The only difference between the two constructs is that the non-codon optimised bxb1 has a weaker RBS, with a strength of 251.82au (translation rate), as calculated by the [Salis RBS Calculator]. Due to this difference in construction we compared each bxb1 to it's own control (see above, Experimental Setup).
We could show that the codon optimised bxb1 could reach ~1/6th flipping compared to control, whereas the non-codon optimised one reached only ~1/260th of its own control. We thus argue that the codon optimised bxb1 is more efficient, especially since the fold increase in efficiency is a lot more than the fold increase in RBS strenght.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 192
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 466
Illegal XhoI site found at 553 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1105
Illegal NgoMIV site found at 1192
Illegal AgeI site found at 242 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1300