Difference between revisions of "Part:BBa K863104"
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K863104 short</partinfo> | <partinfo>BBa_K863104 short</partinfo> | ||
Cellulose binding Domain from Cellulomonas Fimi with const. Promotor J61101 and Reporter GFP | Cellulose binding Domain from Cellulomonas Fimi with const. Promotor J61101 and Reporter GFP | ||
| + | [[Image:Bielefeld2012_pfam00553.jpg|200px|thumb|right|Figure 1: Predicted structure of the CBM_3-family made with Cn3D]] | ||
| + | Cellulose binding domain of the [http://www.ncbi.nlm.nih.gov/nucleotide/327179207?report=genbank&log$=nucltop&blast_rank=3&RID=152ZCN0E01N ''Cellulomonas fimi'' ATCC 484 exoglucanase gene] in Standard 25 (Freiburg) under the constitutive Promotor J23100 with the RBS J61101. This construct was designed as a reporter-fusion-protein to measure the binding capacity of the cellulose binding domain. A GFP is introduced to the C-terminal end of the CBD and connected by a short linker, consisting of four amino acids (Glycine, Serine, Threonine, Glycine). | ||
| + | |||
| + | To that point expression of the protein could not be proven. | ||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
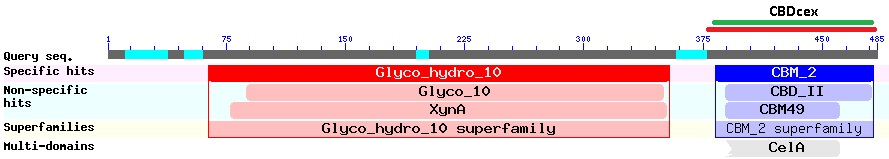
| + | The CBDcex is an C-terminal domain at the end of the protein-sequence (Figure 2). The NCBI-BLAST identified 100 amino acids (300 bases; green bar) as the protein domain. It belongs to the Cellulose Binding Module family 2 (pfam00553/cl02709; Figure 1) and is part of of the Cellulomonas fimi ATCC 484 exoglucanase gene. In our project the domain was used N-terminal, which made the conserved linking sequence to the glycosyl hydrolase unattractive as a Linker for our fusion-proteins. | ||
| + | For the coding sequence 12 bases (4 amino acids) more than the BLAST predicted were taken as conserved sequence upstream and 9 bases (3AS) downstream of the domain to secure natural folding of the domain. | ||
| + | [[image:Bielefeld2012_Cfimiexo.jpg|800px|thumb|center|Figure 2: protein-Blast of the [http://www.ncbi.nlm.nih.gov/nucleotide/327179207?report=genbank&log$=nucltop&blast_rank=3&RID=152ZCN0E01N ''Cellulomonas fimi'' ATCC 484 exoglucanase gene] (the origin of the CBDcex)]] | ||
| + | The expressed GFP-fusion-protein would have 358 amino acids with a molecular weight of 39.1 kDa and a theoretic pI of 6.26. | ||
| + | If the protein concentration is measured by optical density at 280 nm the extinction coefficients would be 49640 M<sup>-1</sup> cm<sup>-1</sup>, assuming all pairs of Cys residues form cystines and 49390 M<sup>-1</sup> cm<sup>-1</sup>, assuming all Cys residues are reduced. The [http://web.expasy.org/cgi-bin/protparam/protparam ExPASy Prot-Parameter-tool] predicted the estimated half-life 30 hours in mammalian reticulocytes, (in vitro) more than 20 hours in yeast (in vivo) and more than 10 hours in ''Escherichia coli'' (in vivo). | ||
| + | It classified the protein as stable. | ||
| + | |||
| + | The GFP used is (mut3b) [note that this part does not have a barcode] | ||
| + | [[Help:Tag|His-Tag]]ged version of gfp from Repressilator reporter. See the [[Part:BBa_E0040:Design | design page]] for more source information. | ||
| + | |||
| + | ====Fluorescence wavelengths==== | ||
| + | Cormack ''et al''.<cite>Cormack</cite> report the following excitation and emission data for GFPmut3 - <br> | ||
| + | *'''Excitation max''' - 501nm | ||
| + | *'''Emission max''' - 511nm | ||
| + | |||
| + | ====Latency==== | ||
| + | Cormack ''et al''.<cite>Cormack</cite> report detectable fluorescence within 8 mins. | ||
<!-- --> | <!-- --> | ||
Revision as of 20:22, 26 September 2012
Cellulose binding Domain from C. Fimi with const. Promotor and GFP
Cellulose binding Domain from Cellulomonas Fimi with const. Promotor J61101 and Reporter GFP
Cellulose binding domain of the [http://www.ncbi.nlm.nih.gov/nucleotide/327179207?report=genbank&log$=nucltop&blast_rank=3&RID=152ZCN0E01N Cellulomonas fimi ATCC 484 exoglucanase gene] in Standard 25 (Freiburg) under the constitutive Promotor J23100 with the RBS J61101. This construct was designed as a reporter-fusion-protein to measure the binding capacity of the cellulose binding domain. A GFP is introduced to the C-terminal end of the CBD and connected by a short linker, consisting of four amino acids (Glycine, Serine, Threonine, Glycine).
To that point expression of the protein could not be proven.
Usage and Biology
The CBDcex is an C-terminal domain at the end of the protein-sequence (Figure 2). The NCBI-BLAST identified 100 amino acids (300 bases; green bar) as the protein domain. It belongs to the Cellulose Binding Module family 2 (pfam00553/cl02709; Figure 1) and is part of of the Cellulomonas fimi ATCC 484 exoglucanase gene. In our project the domain was used N-terminal, which made the conserved linking sequence to the glycosyl hydrolase unattractive as a Linker for our fusion-proteins. For the coding sequence 12 bases (4 amino acids) more than the BLAST predicted were taken as conserved sequence upstream and 9 bases (3AS) downstream of the domain to secure natural folding of the domain.
The expressed GFP-fusion-protein would have 358 amino acids with a molecular weight of 39.1 kDa and a theoretic pI of 6.26. If the protein concentration is measured by optical density at 280 nm the extinction coefficients would be 49640 M-1 cm-1, assuming all pairs of Cys residues form cystines and 49390 M-1 cm-1, assuming all Cys residues are reduced. The [http://web.expasy.org/cgi-bin/protparam/protparam ExPASy Prot-Parameter-tool] predicted the estimated half-life 30 hours in mammalian reticulocytes, (in vitro) more than 20 hours in yeast (in vivo) and more than 10 hours in Escherichia coli (in vivo). It classified the protein as stable.
The GFP used is (mut3b) [note that this part does not have a barcode] His-Tagged version of gfp from Repressilator reporter. See the design page for more source information.
Fluorescence wavelengths
Cormack et al.Cormack report the following excitation and emission data for GFPmut3 -
- Excitation max - 501nm
- Emission max - 511nm
Latency
Cormack et al.Cormack report detectable fluorescence within 8 mins.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal XbaI site found at 37
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal XbaI site found at 37
- 25INCOMPATIBLE WITH RFC[25]Illegal XbaI site found at 37
Illegal NgoMIV site found at 65
Illegal AgeI site found at 1136 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1047