Part:BBa_K863010
tthl laccase from Thermus thermophilus with T7 promoter, RBS and His-tag
tthl (Laccase from Thermus thermophilus) with T7, RBS and HIS tag
Usage and Biology
Slovenia HS characterized this part in 2015.
Escherichia coli BL21 (DE3) bacteria were transformed with the expression plasmids (BBa_K863005 and BBa_K863010) and grown in 10 ml at 37 °C in LBC medium overnight. To express both recombinant proteins, 10 ml of overnight cultures shaker cultures were grown at 37 °C in LB broth supplemented with 30 µg/ml chloramphenicol and shaking with 225 rpm. Expression of the recombinant protein was induced by addition of IPTG to a final concentration of 1 mM, when the cell density reached an OD600 of 0.8. After induction, cells were grown for 5 h and then collected by centrifugation at 6000g for 10 min. The cell pellet collected from 400 ml of bacterial culture was resuspended in 20 ml of resuspension buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 20 mM imidazole) and sonified 3 × 6 min on ice. Following centrifugation at 30 000 × g for 10 min to remove insoluble debris, the supernatant was applied to a Ni-NTA Superflow Cartridge (Qiagen) connected to ÄKTA FPLC system, washed with the resuspension buffer and eluted in the same buffer, but containing 250 mM imidazole. The peak fractions were collected and 15 µl of each fraction was collected and resolved on 12 % SDS-PAGE.


We found that while BBa_K863005 shows excellent activity, BBa_K863010 shows no activity under the same conditions.
IPTG Induction of K863010 (2019 PuiChing_Macau)

Team TecCEM Characterization
TecCEM Characterization, purification and degradation of dyes
For
the characterization of the Laccase BBa_K863010 we conducted an IPTG induction
experiment in which we transformed the plasmid pSB1C3 containing the Laccase in
E. coli BL21-DE3. We thought that we could use another strain called SoluBL21 but results were not successful as no expression
was found. We verified the presence of the protein through an SDS-PAGE with a
gel concentration of 12% and found a visible band with a mass of around 50 kDa. We also analyzed soluble and insoluble fractions and
the presence of protein in the culture medium. This can be seen in figure
1.
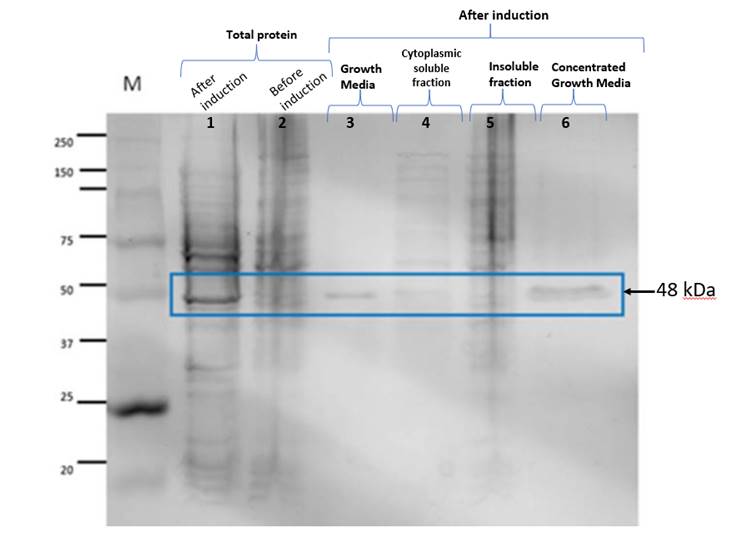
Figure 1. The lanes correspond to
the following. M: Molecular weight marker; 1: Total protein after induction; 2:
Total protein before induction; 3: Protein found in the Culture Medium after
induction; 4: Cytoplasmic soluble fraction; 5: Inclusion bodies of the
insoluble fraction; 6: Concentrated Culture Medium after induction. The band
observed in lanes 1, 3 and 6 weighs around 48 kDa and
corresponds to the expected size.
We
found that the protein was mostly found on the culture medium but can also be
found on the cytoplasmic soluble fraction. The band that was appreciated in
figure 1. indicates that there’s an expression of the Laccase after it’s
induction with IPTG so our results and experience using this part was different
from what 2019 PuiChing Macau’s team reported
previously, since they found no expression and a lack of an IPTG functional
protein.
To
demonstrate activity of the lacasse purified, we
conducted a dye degradation assay. Owing to their broad substrate specificity,
laccases have attracted considerable interest in terms of applications to many
fields, such as environmental detoxification. Some fungal laccases have been
reported to perform dye decolorization of a variety of dyes, such as azo,
anthraquinone, and aromatic methane. Synthetic dyes are widely used in several
industries, including textiles, food processing, paper printing, cosmetics, and
pharmaceuticals. Three dyes were selected according to previous reports of
laccase degradation, malachite green, methylene blue and rose Bengal (Cheriaa J. & Bakhrouf A.,
2009 [1]; Forootanfar, H., 2012; Pramanik,
S., & Chaudhuri, S. 2018 [2]). Based on the chemical structure of
chromogenic groups, dyes are classified as azo, heterocyclic/polymeric or
triphenylmethanes. About 60% of produced dyes belong to the azo group which are
categorized as monoazo, diazo, and triazo dyes. Malachite green is a triphenylmethane dye
belonging to a basic dyes class, used extensively for dyeing silk, wool and cotton. Rose Bengal, an Azo dye is used in
apoptosis assays, biological staining photography, recording industry, etc.,
this dye is genotoxic and microbial toxic. Methylene blue is a heterocyclic
aromatic compound that has a wide use in biological and medicine applications
in addition to textile-processing industries. To prove that the Laccase was
being expressed, we conducted a dye degradation involving three colorants that
act as a substrate: methylene blue, malachite green and rose bengal. We based this experiment upon the findings of D.
Singh et al. (2014) [3], in which they used agar plates with these colorants to
determine the expression of Laccases in a medium. The first assays we conducted
were only to find out if there was any color change with the presence of our
extracted Laccase either from the cytoplasmic soluble fraction or from the
culture medium. We used Citrate Buffer and found a change of color in different
samples of Laccase after its purification using Ni Affinity. We also verified
that the effect we saw wasn’t related to a change in pH.
The results are available in figure 2.
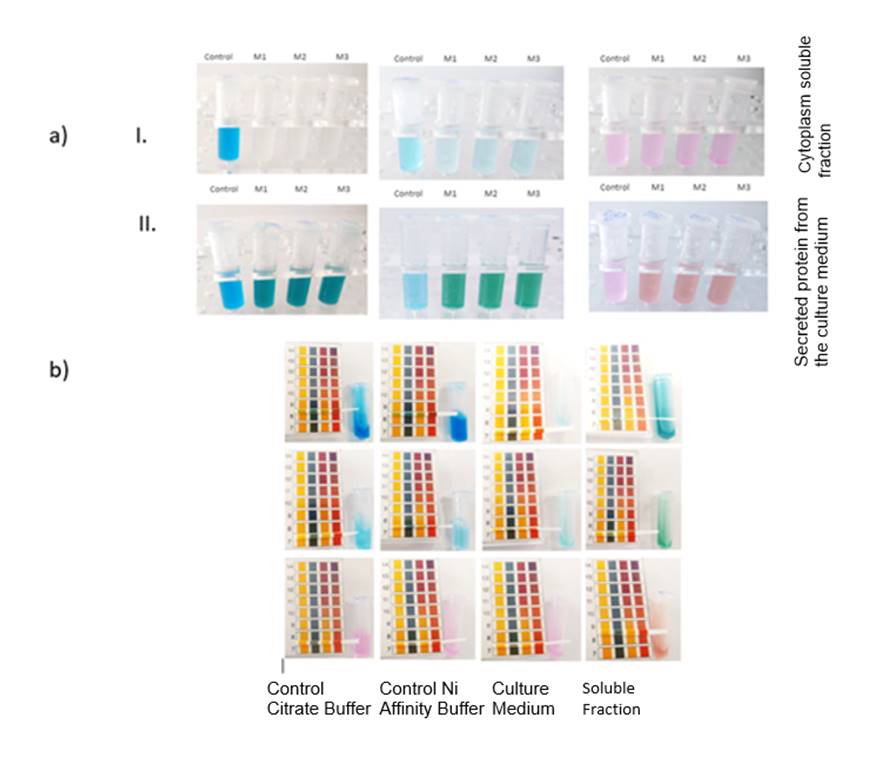
Figure 2. First dye degradation
assay conducted. In section a) we can see the change of color of the substrates
used (methylene blue, malachite green and rose bengal
from left to right). a)-I. corresponds to the cytoplasm soluble fraction while
a)-II. corresponds to the secreted protein from the culture medium. In section
b) we can see that the pH remained unchanged throughout the assay.
After
this first assay, we decided that we had to establish a purification protocol
through which we could get the most enzyme possible. We used a Ni Affinity
Column and a system of recollection of the different phases. We collected the
enzyme from both the culture medium and the cytoplasmic soluble fraction and
measured the absorbance of the fractions collected at 280 nm. In total, we got
21 fractions for the cytoplasm proteins and 20 fractions for the culture
medium. The purification conditions were established using a growing elution
buffer concentration. These conditions
are shown in figures 3 and 4.

Figure 3. Chromatogram of the purification
of the cytoplasmic soluble fraction showing the spike of absorbance in an
elution volume of around 15 mL to 20 mL (with a percentage of elution buffer of
around 30 to 50%); corresponding to the fractions containing the Laccase.
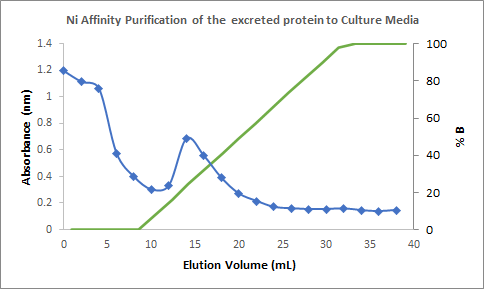
Figure 4. Chromatogram of the
purification of the culture medium showing the spike of absorbance in an
elution volume of 12 mL to 20 mL (with a percentage of elution buffer of around
16% to 50%); corresponding to the fractions containing the Laccase.
Finally,
we conducted a last assay in which we first quantified the amount of protein
recovered through a BCA quantification protocol. Using this protocol and with
the elaboration of a BCA curve, we estimated that we recovered 0.334 µg/mL of
Laccase in the culture medium while for the cytoplasmic soluble fraction we
obtained 0.1298 µg/mL of Laccase. This was consistent with the results we got
from the chromatograms.
For
the final colorimetric assay we conducted, we quantified the activity of the
Laccase obtained from the purification. We compared it with a Commercial
Laccase from Sigma belonging to Trametes
versicolor and used the spectrophotometer to measure methylene blue,
malachite green and rose bengal at 664, 617 and 562
nm respectively. Since we didn’t quite have the exact concentration of
colorants in our samples, we limited to measure the activity as a percentage of
degradation of each colorant where a 100% of substrate would be the absorbance
of the control of the blue, green and rose colorants
and the enzymatic degradation would be expressed as the loss of color. The results can be seen below.
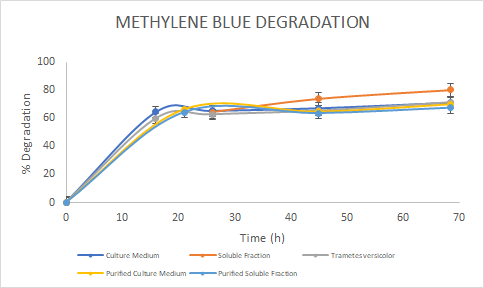
Figure 5. Percentage of
degradation of Methylene Blue through time for the Laccase in the culture
medium (blue), soluble fraction (orange), purified culture medium (yellow),
purified soluble fraction (light blue) and the Laccase from Trametes
versicolor (gray).
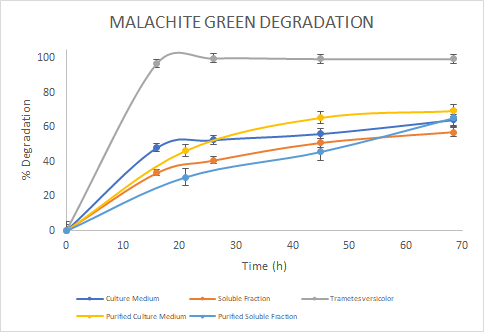
Figure 6. Percentage of
degradation of Malachite green through time for the Laccase in the culture
medium (blue), soluble fraction (orange), purified culture medium (yellow),
purified soluble fraction (light blue) and the Laccase from Trametes
versicolor (gray).
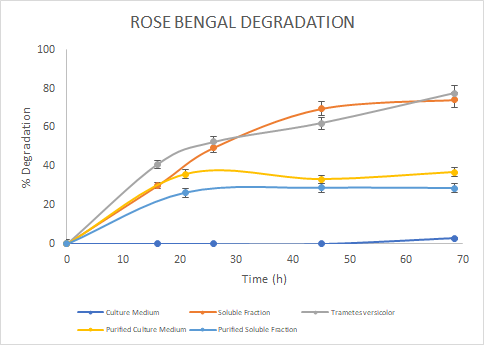
Figure 7. Percentage of
degradation of Rose Bengal through time for the Laccase in the culture medium
(blue), soluble fraction (orange), purified culture medium (yellow), purified
soluble fraction (light blue) and the Laccase from Trametes
versicolor (gray).
In
the assays, we saw a similar trend for the degradation of Methylene Blue. For
Malachite Green, the Laccase from Trametes versicolor
had a higher degradation rate. Finally, for Rose Bengal we observed a similar
trend between Trametes versicolor Laccase
and the Soluble Fraction Laccase (before purification). We then reported the
percentage of degradation of each sample for each colorant at the final point
in time and got the next results:

Figure 8. Percentage of
degradation of each Laccase at the final point in time for each colorant.
We
observed that overall, the best results were obtained by the Laccase of Trametes versicolor (as expected) followed by
the soluble fraction and the purified soluble fraction. However, it is worth
noting that although these results show that the commercial Laccase may have
higher degradation values, it also has a higher concentration since it was
prepared at 1 mg/mL (compared to the purified Laccases which had concentrations
of 0.334 µg/mL in the culture medium and 0.1298 µg/mL in the soluble
fraction.
In
conclusion we found that the best degradation profile was that of dye malachite
green which was degraded almost 100% with the commercial Laccase. However, the
most consistent results were obtained from methylene blue and show a similar
trend of its percentage of degradation for every fraction analyzed. We saw that
Laccases that come from different sources may have a different efficiency of
degradation for different substrates, hence the importance of characterizing
them.
References:
[1] Cheriaa, J., & Bakhrouf, A. “Triphenylmethanes,
malachite green and crystal violet dyes decolourisation
by Sphingomonas paucimobilis”. Annals
of microbiology, 59(1), 57-61. 2009
[2] Forootanfar, H., Moezzi, A., Aghaie-Khozani, M., Mahmoudjanlou,
Y., Ameri, A., Niknejad, F., & Faramarzi, M. A. “Synthetic dye decolorization by three
sources of fungal laccase”. Iranian journal of environmental health
science & engineering, 9(1), 1-10. 2012.
[3] D.
Singh et al., “Isolation, Characterization and Production of Bacterial Laccase
from Bacillus sp”, 06 2014, bll
439–450. 2014.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 1408
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 475
Illegal NgoMIV site found at 962 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 790
//function/degradation
//proteindomain/degradation
| None |
