Part:BBa_K4130000
Contents
- 1 IgG F(c) Binding Protein, EibD
- 1.1 Biology
- 1.2 Usage
- 1.3 Sequence and Features
- 1.4 Characterization
- 1.4.1 Induction of EibD Expression Results in Whole-Cell Autoaggregation Phenotype
- 1.4.2 EibD Expressed on Live Cells Binds FITC-Tagged IgG
- 1.4.3 Reduction in Autoaggregation Upon Addition of Antibodies
- 1.4.4 Biosensor for GFP Differentiates Between GFP Concentrations
- 1.4.5 Absence of Response to Addition of BSA-Asparagine "Beads"
- 1.4.6 Biosensor for Asparagine Differentiates Between Asparagine Concentrations
- 1.5 Updated Model of Biosensor Mechanism
- 1.6 Conclusions
- 1.7 References
IgG F(c) Binding Protein, EibD
Biology
Escherichia coli Ig-binding proteins (Eibs) are a wide-spread class of proteins within intimin-negative, shiga-toxin producing enterohemorrhagic E. coli (EHEC) strains [1]. First discovered in strain ECOR-9 [2, 3], the class has grown to include EibA, C, D, E, F, and G. The class is united in its common characteristic of immunoglobulin (Ig) binding activity.
Immunoglobulins, also known as antibodies, are a family of heterodimeric proteins produced by B cells capable of binding with great specificity to respective antigens. Igs are typically composed of a “variable region”, responsible for binding of the antigen, and a “constant region” with no affinity for the antigen. Igs are further classified according to their “constant regions”, into 5 classes: IgM, IgG, IgA, IgD, and IgE. [4]
E. coli Ig-binding proteins have been shown to be capable of binding the constant region of assorted Ig classes. Affinity and specificity for different classes of Ig proteins varies between Eib family members, with dissociation constants on the 100 nM scale [5].
EibD (from E. coli strain ECOR-9) is a 511 amino acid, 210 kDa [3] member of the Eib family. The protein exhibits affinity toward both species-nonspecific IgG (Kd = ~73 nM) and IgA (Kd = ~135 nM) [5], with separate binding domains for each Ig [6]. The structure also includes a membrane anchor, left-handed coiled-coil, saddle domain, right-handed coiled-coil, neck, and head domain [6], characteristic of trimeric autotransporter adhesins - a family of secreted proteins in Gram-negative bacteria that are associated with virulence [7]. In addition to Ig-binding properties, heterologous expression of EibD has resulted in whole-cell self-aggregation phenotypes [6], typical of other trimeric autotransporter adhesins such as YadA [7]. Transmission electron microscopy (TEM) imaging has revealed zipper-like structures forming between neighboring bacteria harboring EibD, explaining the autoaggregation phenotype. Although the biological function of EibD is unknown, its Ig-binding properties and homology to other autotransporter adhesins implicates its role in serum resistance; binding of IgG by EibD may block binding of immunoglobulins to the adaptive immunity protein C1q, protecting bacteria against innate host defenses [6].
Usage
A major avenue of synthetic biology research is the development of whole-cell bacterial biosensors: bacteria that have been genetically engineered to “sense” and “report” the concentration of molecules of interest. These biosensors represent a promising improvement to current biosensors, due to their low-cost, self-manufacturing, and biodegradable properties. However, advancements in whole-cell bacterial detection remain hindered by difficulties associated with having to re-engineer a new solution for each compound of interest. In response to this challenge, we have designed an EibD-based universal biosensor. We chose EibD for its Ig (antibody)-binding properties. Expression of EibD results in auto-agglutination and biofilm formation that can be observed via the eye [6]. By incubating EibD-expressing bacteria with immunoglobulin antibodies, we hoped to be able to detect various antigens. Introduction of the antibodies would cause visual and quantitative dis-agglutination by molecular competition between self-self interactions and self-antibody interactions. Addition of the antigen would lead to multivalent binding interactions (multiple antibody-bound bacteria binding the same antigen), causing re-agglutination of the cells. (Figure 1)
Accordingly, this strategy of detection has the potential to be applied to nearly any antigen with zero need for further genetic engineering. Previous whole-cell bacterial sensors have been focused on expressing antibodies or antibody fragments on the surface of the bacterium, meaning that each strain is specific to an antigen. By expressing a general antibody-binding protein, a single strain can be utilized for the detection of multiple antigens.
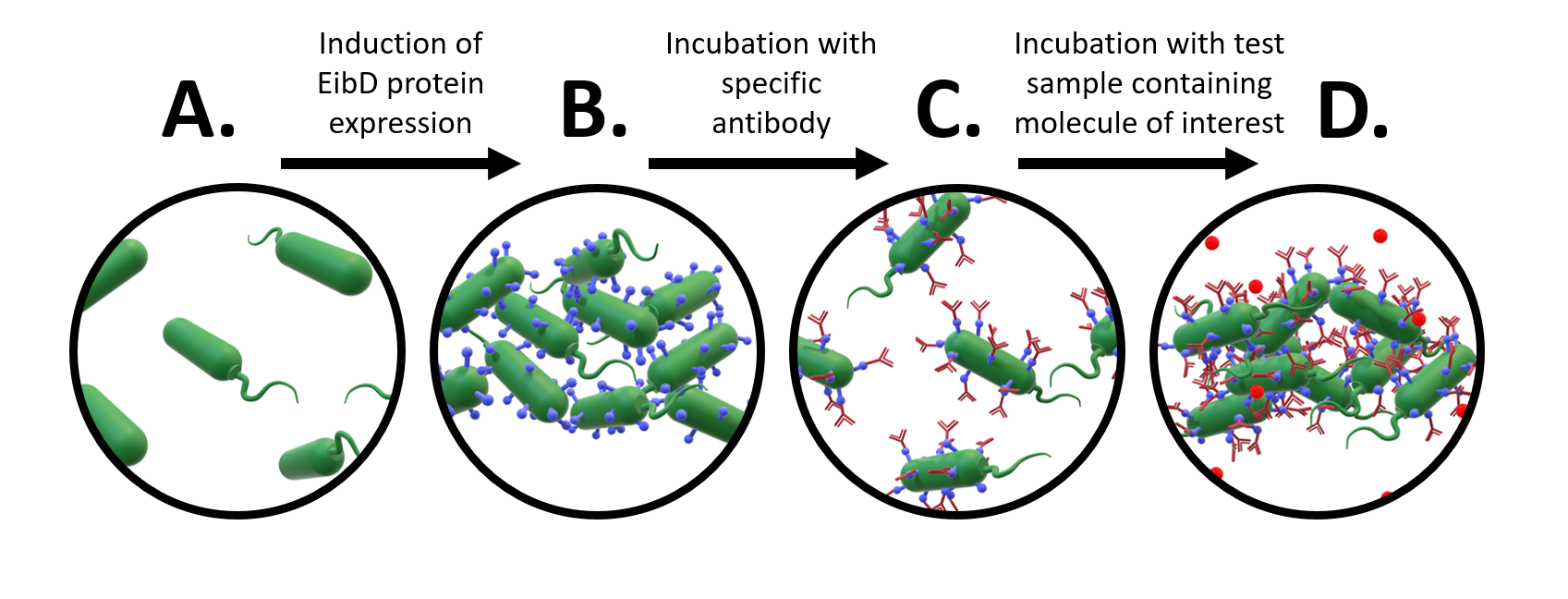
Figure 1: Proposed Design of Universal Whole-Cell Biosensor. a) uninduced E. coli exhibit relatively few interactions b) after induction of EibD expression, bacteria begin to "autoaggregate", forming clumps due to homophilic EibD-EibD interactions c) incubation with an antibody reduces bacterial autoaggregation due to competition between EibD-antibody and EibD-EibD binding interactions d) after incubation with a sample containing the antigen, multivalent interactions form, where multiple antibodies bind to the same antigen. This results in an increase in autoaggregation.
In keeping with the overarching goal of our project, we chose to exploit the design of our universal whole-cell biosensor for the detection of the small molecule asparagine. Unlike larger molecules such as proteins, asparagine presents a unique challenge in that it contains limited theoretical binding sites for antibodies. We therefore had to make modifications to the design of our biosensor to accommodate monovalent antibody-antigen interactions. In this modified design, a non-antigenic protein is crosslinked to multiple small molecules of interest, forming an antigenic "bead". This "bead" can be used to induce multivalent antibody-antigen interactions, similar to what happens in the case of a larger molecule of interest. These multivalent interactions can subsequently be competed out by free antigen present in a test sample. (Figure 2)

Figure 2: Application of EibD whole-cell biosensor to detecting amounts of free asparagine in maple syrup. A) Uninduced E. coli B) EibD-expressing bacteria exhibiting autoaggregating phenotype C) Reduction in autoaggregating phenotype after incubation with anti-asparagine antibody D) Addition of BSA-Asparagine beads that allow for bivalent interactions between the antibodies, causing agglutination (re-clumping) of the bacteria E) Addition of "test sample" containing free asparagine causes molecular competition for binding free asparagine and bead-bound asparagine. This results in a reduction in agglutination proportional to the concentration of free asparagine competitor.
We therefore designed a genetic circuit composed of the EibD gene from E. coli strain ECOR-9, a rhamnose-inducible promoter (BBa_K914003), strong ribosome binding site (BBa_B0034), and double-terminator (BBa_B0015). Synthesized parts were assembled into the chloramphenicol-resistance conferring pSB1C3 vector.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 1482
- 1000COMPATIBLE WITH RFC[1000]
Characterization
The EibD biobrick was assembled into the pSB1C3 vector by 3A assembly and cloned into E. coli DH5a for storage and E. coli BL21 for expression experiments.
Induction of EibD Expression Results in Whole-Cell Autoaggregation Phenotype
Cultures of E. coli BL21 (+/- biobrick BBa_K4130000) were grown to mid-exponential phase (O.D.600 ~ 0.4 - 0.6), where growth and protein translation is maximal. Subsequently, to determine the differential effect of inducer concentration, bacteria were induced with 0.0% rhamnose, 0.001% rhamnose, or 0.01% rhamnose and grown for 2 hours at 30 C, 200rpm. After induction, the EibD-transformed bacterial cultures appeared markedly different from a BL21 control strain harboring no plasmid. A white precipitate accumulated on the bottom of the EibD cultures induced with 0.001% L-rhamnose, while the uninduced EibD culture exhibited no formation of white precipitate (Figure 3). This white precipitate is likely a result of autoaggregation due to previously reported homophilic interactions between EibD proteins on separate bacteria [6]. To investigate this, we used light microscopy to visualize the bacteria. Bacterial cultures were pelleted and washed in PBS, followed by incubation with acidic proteoglycan-staining safranin (Figure 4). Control E. coli BL21 without the EibD-containing plasmid appear evenly dispersed throughout the field of view. In contrast, EibD-expressing E. coli BL21 appear to aggregate in clumps within the field of view. This evidence is further indicative of autoaggregation. We quantified the degree of autoaggregation by measuring the settling rate of bacteria in solution over time. After brief mixing, the optical density at 600 nm (O.D.600) was recorded over a period of 30 minutes. As the bacteria settled and precipitated over time, a decrease in optical density was observed. The O.D.600 was observed to decrease more rapidly with increasing concentrations of L-rhamnose inducer, indicating that autoaggregation correlates positively with induction concentration. (Figure 5)

Figure 3: Visual Observation of Autoaggregation Mid-exponential phase BL21 cultures (with and without EibD-harboring plasmid) were induced with 0% or 0.001% L-rhamnose and grown for 2 hours. 2mL of pelleted bacteria was resuspended in PBS, briefly mixed, and allowed to settle over a period of 70 minutes. Images were taken at 0 minutes, 40 minutes, and 70 minutes.
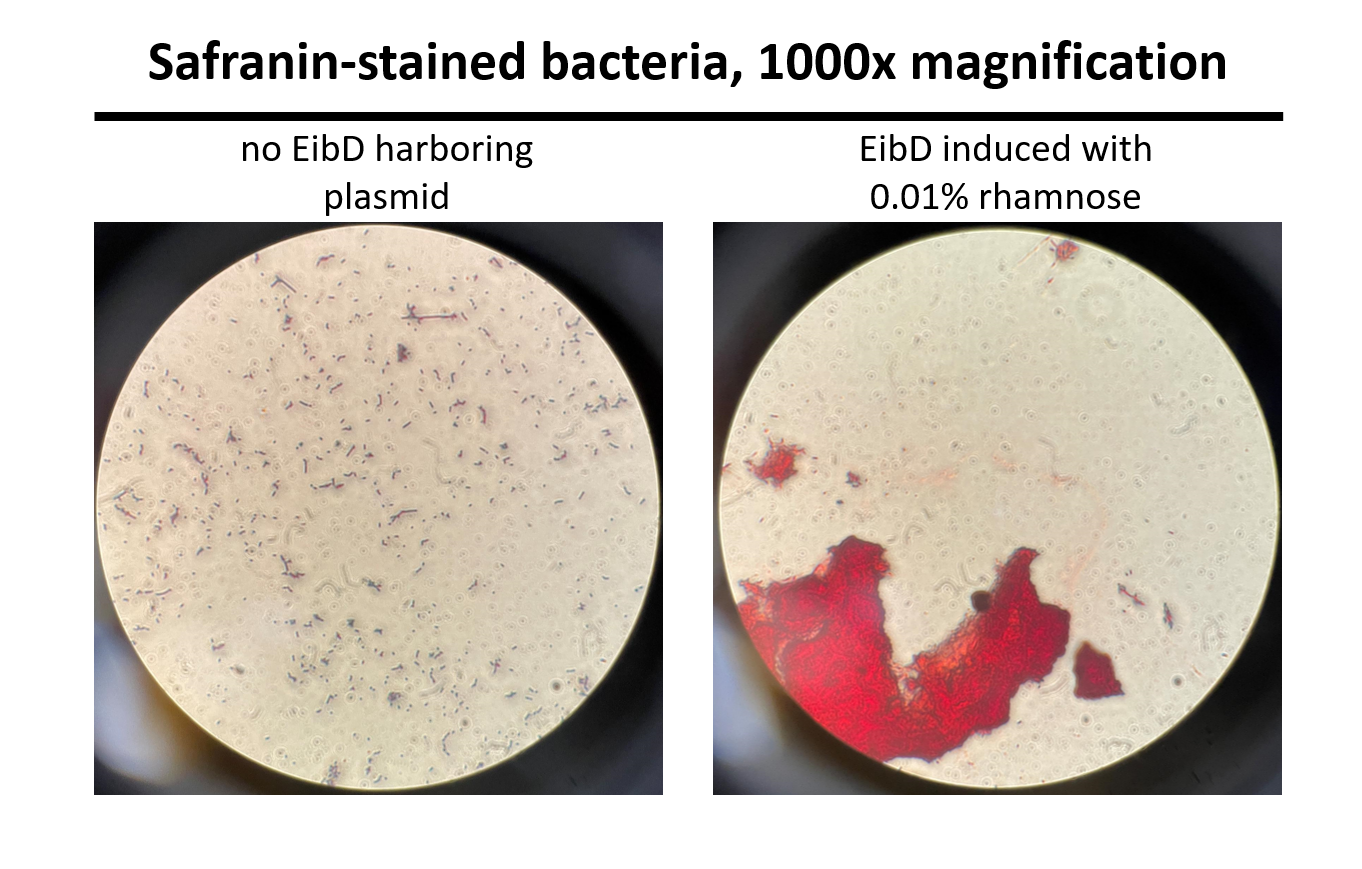
Figure 4: Light Microscopy Observation of Autoaggregation Induced bacteria were stained with safranin and visualized using a light microscope at 1000x magnification. Pictures were taken through the eyepiece using a phone camera. As can be seen on the left, control E. coli BL21 bacteria are evenly distributed throughout the field of view. On the right, EibD-induced bacteria appear to clump together, indicative of autoaggregation.
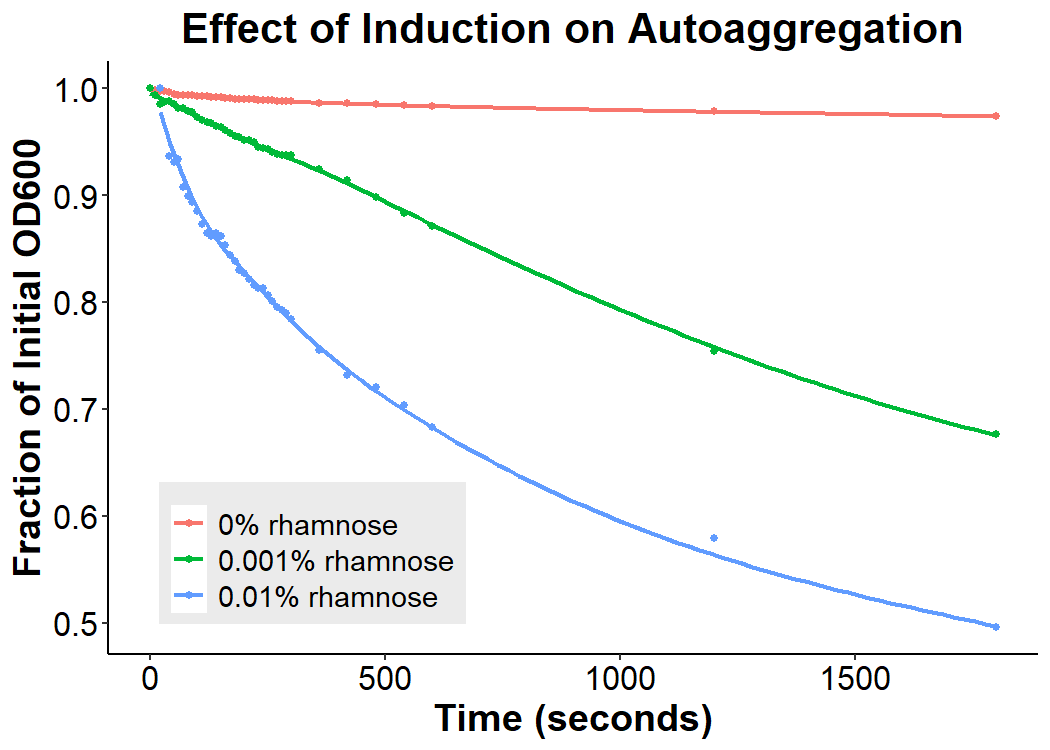
Figure 5: Effect of Induction Concentration on Agglutination Mid-exponential phase BL21 EibD-transformed cultures were induced with 0%, 0.001%, and 0.01% L-rhamnose and grown for 2 hours. 2mL of pelleted bacteria was resuspended in PBS, briefly mixed, and the optical density at 600nm was monitored for 30 minutes.
EibD Expressed on Live Cells Binds FITC-Tagged IgG
EibD has previously been shown to be capable of non-immune binding to IgG [3]. The premise of our biosensor depends heavily upon this capability. Therefore, it was necessary to demonstrate that expression of BioBrick BBa_K4130000 in E. coli BL21 produces functional EibD capable of binding IgG. The EibD-harboring strain was induced with 0%, 0.001%, or 0.01% L-rhamnose and cultured for 2 hours. Bacteria were harvested and washed in PBS two times. Subsequently, bacteria were incubated in 200ug/mL mouse IgG-FITC for 24 hours. Bacteria were pelleted and washed twice, followed by fixation and staining for DNA by NucBlue. (Figure 4).
Comparing Figure 6A-C, as inducer concentration is increased, large clumps of bacteria become apparent. This is suggestive of autoaggregation, and further supports the quantitative data in Figure 5, and qualitative data in Figures 3 and 4.
Comparing Figure 6A and D, B and E, and C and F indicates localization of the IgG-FITC protein and the DNA. Throughout all induction levels, IgG-FITC signal is weak, except for exceedingly bright spots that do not colocalize with the DNA. These bright spots likely represent aggregates of the IgG-FITC protein in solution. The weaker “blots” of FITC fluorescence do, however, co-localize with the DNA stain in Figure 6, panels A-C. This suggests that IgG-FITC may be interacting with the EibD-expressing bacteria as observed in previous research.
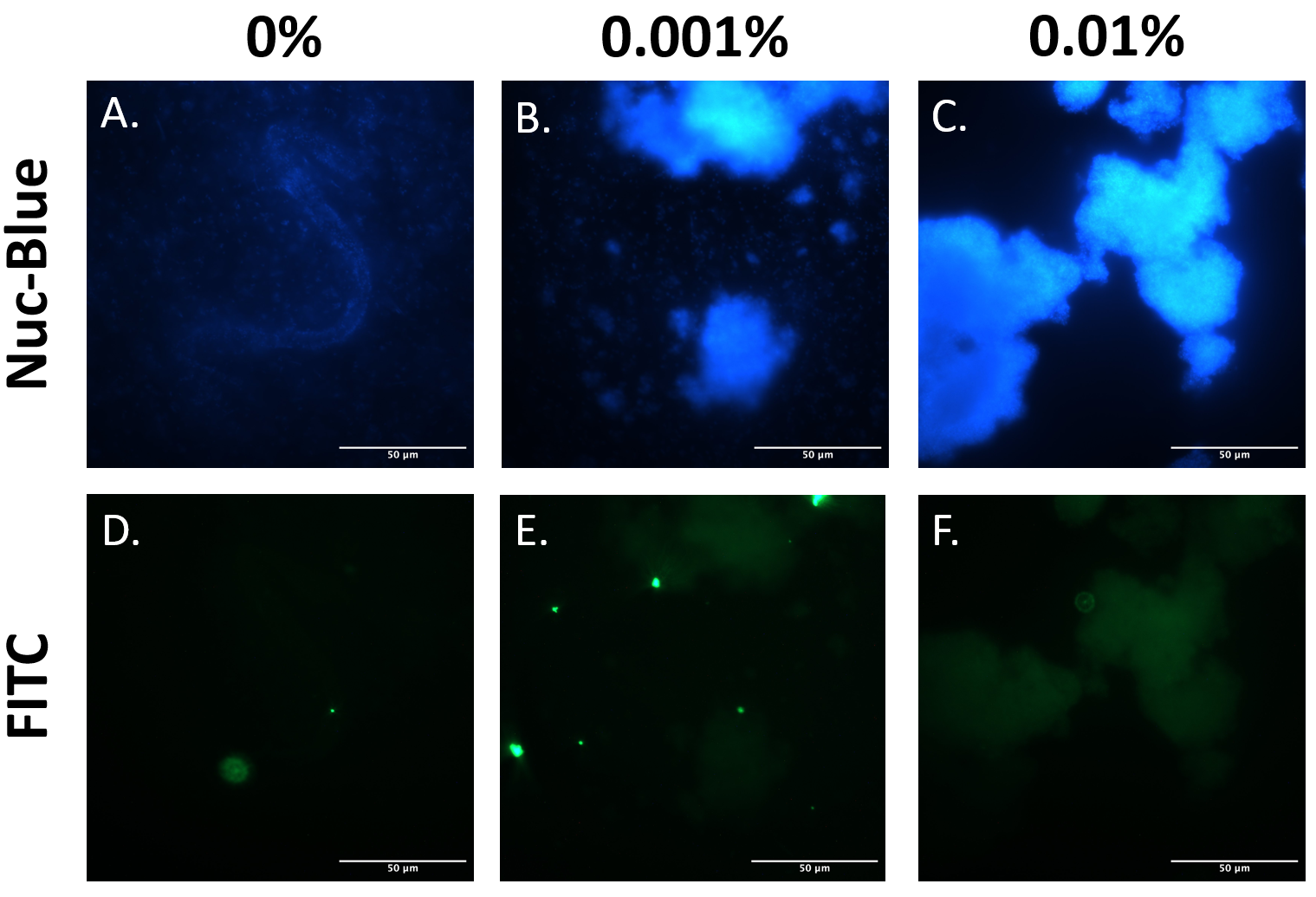
Figure 6: IgG-FITC Co-Localizes With EibD-Induced Bacteria Harvested bacterial cultures induced with 0%, 0.001%, and 0.01% rhamnose were incubated with mouse IgG-FITC for 24 hours and then washed. Fixed bacteria were stained for DNA using NucBlue Fixed Cell ReadyProbes Reagent and imaged. Panels A and D, B and E, and C and F correspond to the same region. .
Reduction in Autoaggregation Upon Addition of Antibodies
In order to impart specificity towards a particular compound into the whole-cell bacterial biosensor, the EibD-expressing bacteria must be incubated with an antibody for the compound of interest. This will allow binding of the EibD protein to the antibody, thus creating a bacterium coated in antibodies. The intended application of our biosensor in the maple syrup industry is to detect buddy maple sap, which is associated with increases in free asparagine. Therefore, we incubated our EibD-expressing bacteria with an anti-asparagine antibody (Immusmol). To test the universality of our biosensor, we also incubated our EibD-expressing bacteria with an anti-GFP antibody.
Figure 7 displays the effect of incubation with the anti-asparagine antibody on autoaggregation. Bacteria were incubated with or without 2 mg/mL antibody in PBS for 20 hours. The bacteria were resuspended briefly and the O.D.600 was measured over 30 minutes. Experiments were performed in triplicate, and the average fraction O.D.600 was calculated for each time point. The error ribbon on the graph represents 1 standard deviation from the mean (n=3). The addition of the anti-asparagine antibody significantly reduced the amount of autoaggregation (Figure 7). This is consistent with the hypothesis that the antibodies may compete with the homophilic interactions between EibD proteins causing autoaggregation. The data suggests that the anti-asparagine antibody has successfully been bound by the EibD protein on the surface of the bacteria. Figure 8 displays the effect of incubation with anti-GFP antibody on autoaggregation. Bacteria were treated identically as the anti-asparagine antibody treated samples (2 mg/mL antibody in PBS for 20 hours). Incubation with the anti-GFP antibody resulted in a significant reduction in autoagglutination. This is consistent with the results of incubation with the anti-asparagine antibody, and suggests that the anti-GFP antibody has successfully been bound by the EibD protein on the surface of the bacteria.
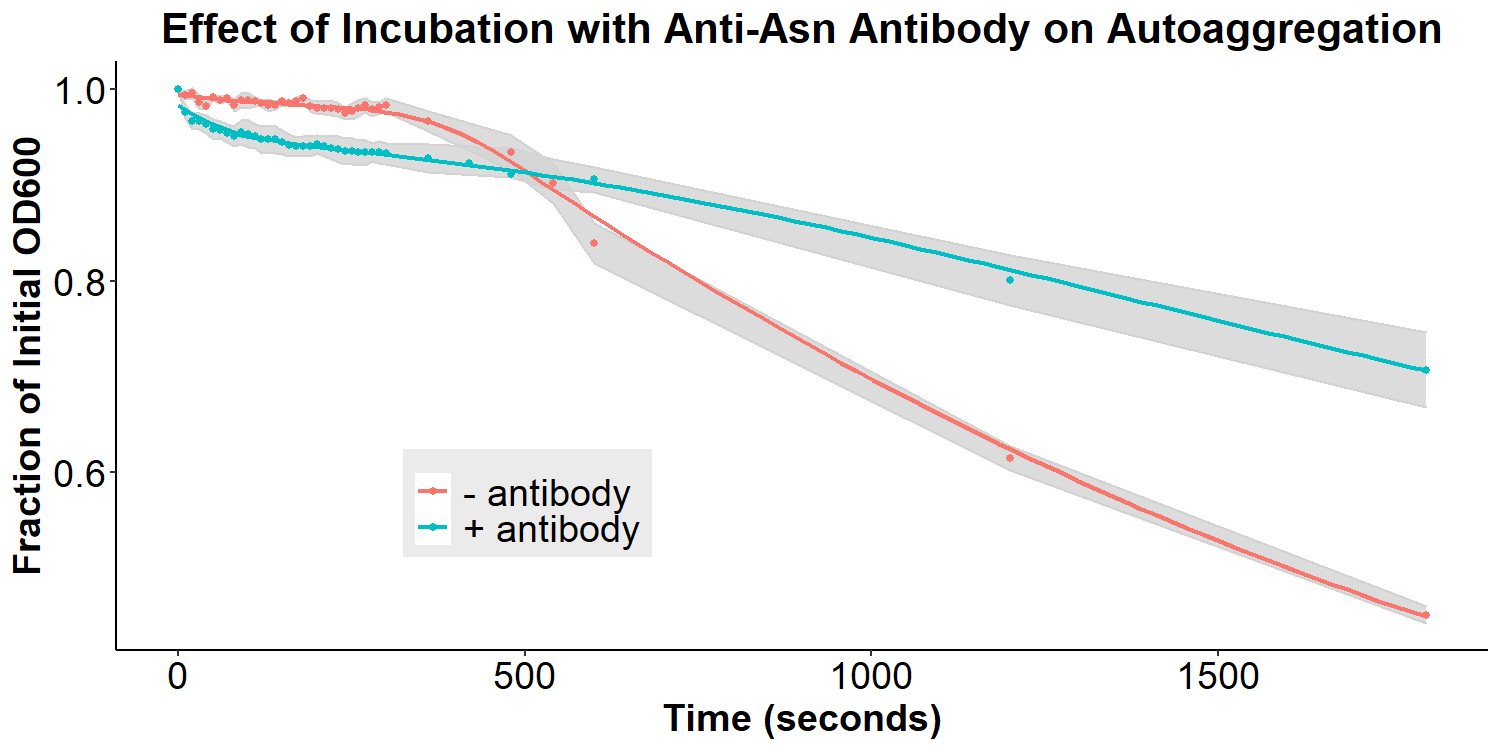
Figure 7: The Effect of Incubation with Anti-Asparagine Antibody on Autoaggregation Bacteria were incubated with or without 2 mg/mL anti-asparagine antibody in PBS for 20 hours. The bacteria were resuspended briefly and the O.D.600 was measured over 30 minutes. Experiments were performed in triplicate, and the average fraction O.D.600 was calculated for each time point. The error ribbon on the graph represents 1 standard deviation from the mean. .
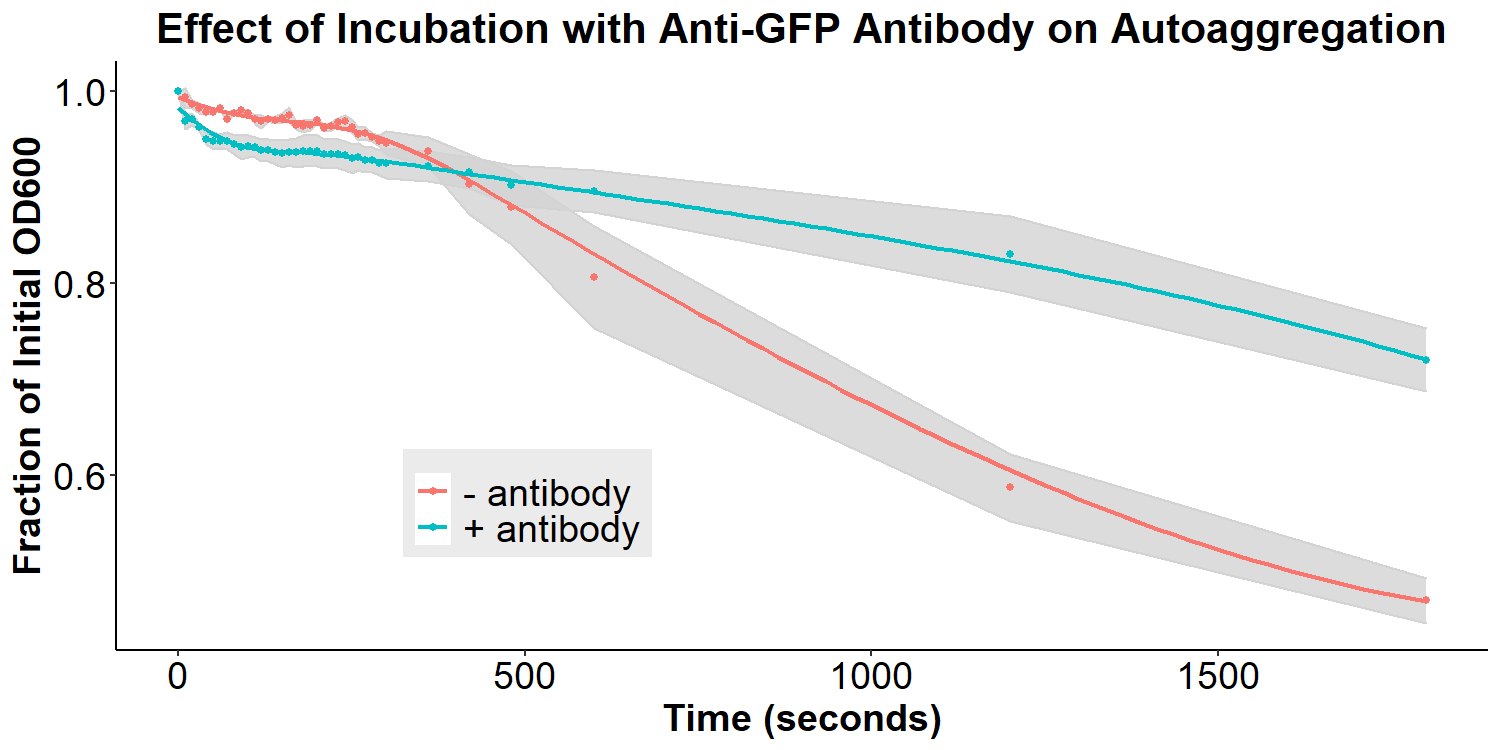
Figure 8: The Effect of Incubation with Anti-GFP Antibody on Autoaggregation Bacteria were incubated with or without 2 mg/mL anti-GFP antibody in PBS for 20 hours. The bacteria were resuspended briefly and the O.D.600 was measured over 30 minutes. Experiments were performed in triplicate, and the average fraction O.D.600 was calculated for each time point. The error ribbon on the graph represents 1 standard deviation from the mean.
Biosensor for GFP Differentiates Between GFP Concentrations
Referencing the schema in Figure 1, the next step in building a biosensor for GFP is to incubate with known concentrations of GFP and observe the resulting autoaggregation. According to our model, due to the size and relative complexity of the structure of GFP, polyclonal antibodies should bind to multiple GFP locations. Since the polyclonal antibodies are attached to the bacteria via EibD, these multivalent antibody-GFP interactions should cause an increase in the clumping/autoaggregation behavior of the bacteria. The amount of autoaggregation should also be a function of the amount of GFP in the system.
To test our model, anti-GFP antibody-coated bacterial cultures were incubated with 0uM, 0.96uM, and 3.85uM GFP in PBS overnight for 16 hours. The cultures were briefly resuspended and the O.D.600 was monitored for 30 minutes. There appears to be an overall decrease in autoaggregation with the addition of any concentration of GFP (Figure 9). However, due to differences in original autoaggregation profile between samples, a more accurate measure of the data is to subtract the fraction O.D.600 after addition of GFP from the fraction O.D.600 from before the addition of GFP (of the same sample). This will eliminate any sample-to-sample variability that may contribute to false interpretations of data. The re-analyzed graph can be seen in Figure 10, displaying that the autoaggregation profile of the samples varies as a function of GFP concentration. When taken together with Figure 9, the data indicates that increasing concentrations of GFP results in decreases in autoaggregation.
Interestingly, the data are not supportive of the hypothesis that addition of GFP will lead to bivalent antibody-GFP interactions, increasing autoaggregation. Therefore, a new molecular model was proposed to better fit the data (Figure 15). In this new model, addition of the antibody does compete away homophilic EibD-EibD interactions. However, as indicated by the data in Figures 5 and 6, this does not result in zero autoaggregation. This may be because of additional aggregation that is imparted by the antibodies themselves. While the EibD proteins may no longer be interacting, the antibodies may be interacting with each other. This is supported by previous research that has indicated that the immunoglobulin Greek-key beta sandwich folding has been shown to be susceptible to edge-edge association [8]. Additionally, the complementarity determining regions of antibodies responsible for binding to antigens are often composed of hydrophobic and electrostatic residues, which can also contribute to aggregation [9,10]. These antibody-antibody interactions may not be as strong as the EibD-EibD interactions, causing a reduction, but not full absence, of autoaggregation. In this new model, addition of the antigen, GFP, may therefore cause a decrease in autoaggregation by out-competing the antibody-antibody interactions and increasing steric hindrance. This new model may explain the decrease in autoaggregation observed in Figures 9 and 10.
Overall, the data from Figure 10 demonstrates that we have been successful in creating a sensitive biosensor for the detection of GFP.
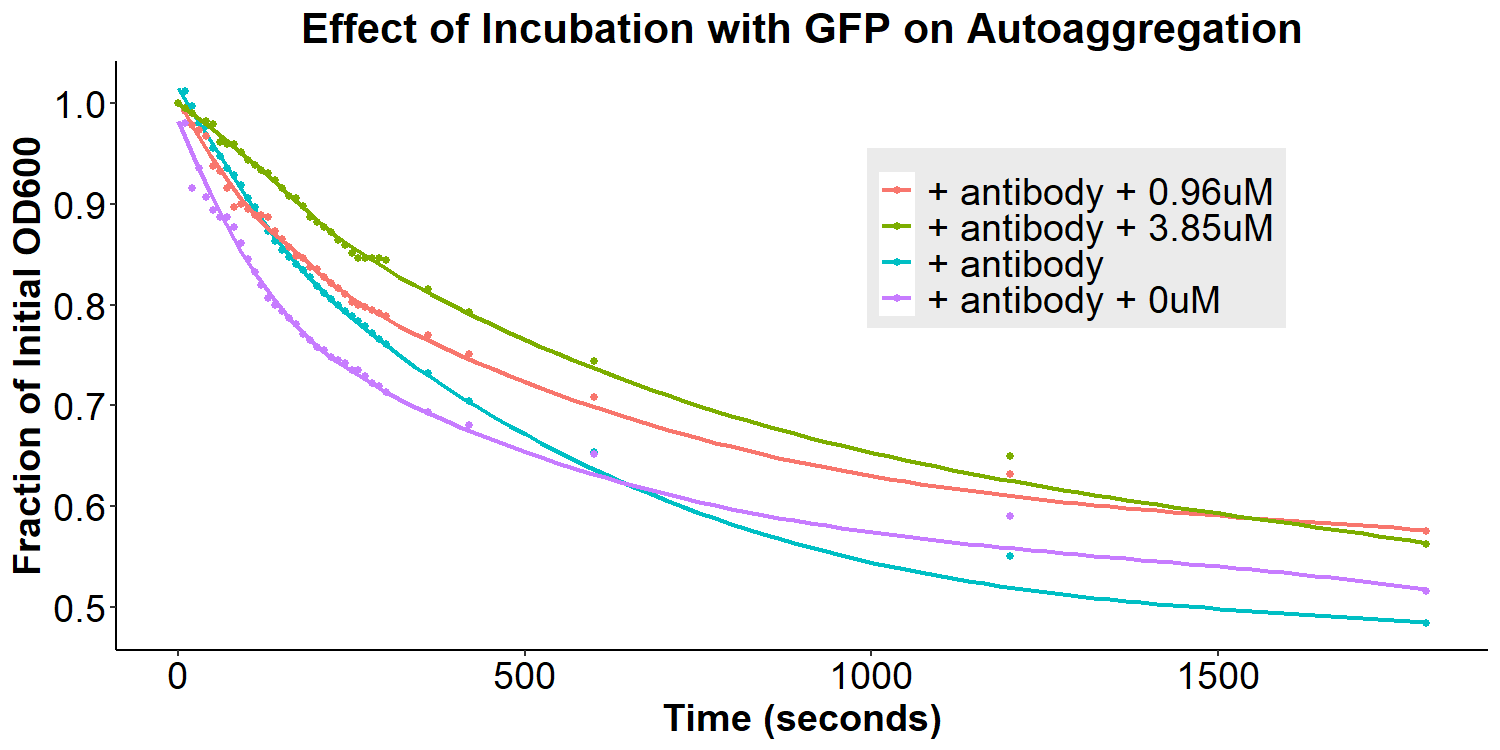
Figure 9: Effect of Incubation with GFP on Autoaggregation Bacterial cultures were incubated with 2mg/mL anti-GFP antibody for 3.5 hours and then washed, removing any unbound antibody. Subsequently, bacteria were resuspended in 0uM, 0.96uM, or 3.85uM GFP and incubated for 12 hours. Bacteria were briefly resuspended and the O.D.600 was measured for 30 minutes.
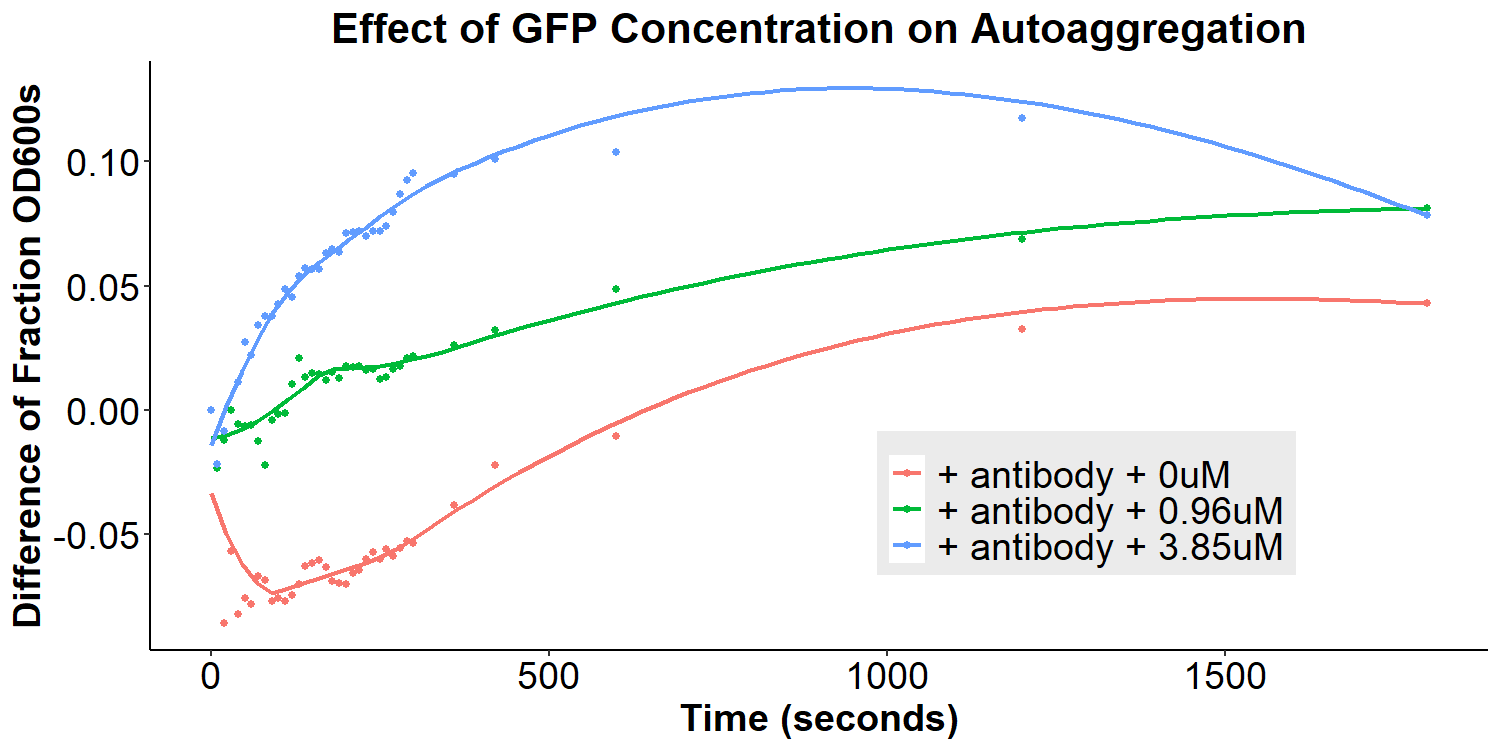
Figure 10: Effect of GFP Concentration on Autoaggregation Bacterial cultures were incubated with 2mg/mL anti-GFP antibody for 3.5 hours and then washed, removing any unbound antibody. The O.D.600 was measured for 30 minutes (Fraction O.D.600 pre-GFP) Subsequently, bacteria were resuspended in 0uM, 0.96uM, or 3.85uM GFP and incubated for 12 hours. Bacteria were briefly resuspended and the O.D.600 was measured for 30 minutes (Fraction O.D.600 post-GFP). The difference between Fraction O.D.600 pre-GFP and post-GFP was calculated and plotted as a function of time for each sample.
Absence of Response to Addition of BSA-Asparagine "Beads"
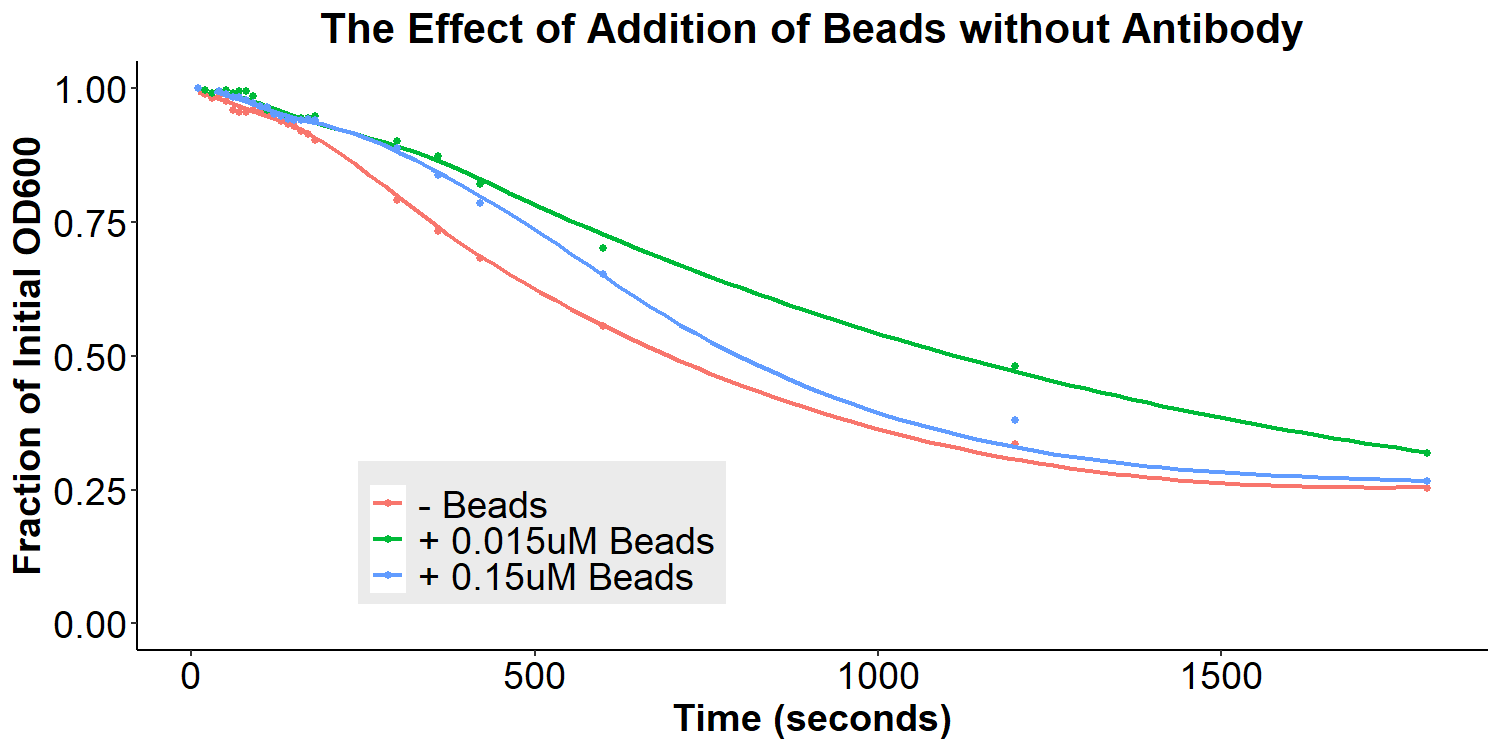
According to the original model (Figures 1 and 2) of our biosensor, BSA-Asparagine “beads” would be necessary to detect a small molecule such as asparagine. Addition of the beads was expected to increase bacterial aggregation due to multiple bacteria binding the same bead. Thus, we designed experiments to examine the aggregative effect of addition of BSA-Asparagine beads (Figures 11 and 12) to the system. We first ran a control experiment to examine the effect of addition of the beads themselves (Figure 11). EibD-expressing bacteria were incubated with 0.015uM and 0.15uM BSA-Asn beads alone. There is a slight dis-aggregation of the bacterial cultures, in a near negligible amount. We next examined the effect of the addition of the beads while in the presence of the antibody-incubated bacteria (Figure 12). According to the original biosensor model, this should cause an aggregation event between the bacteria. However, there is no observable response to the addition of the beads (Figure 12). This could be due to several reasons: 1) the antibody is not recognizing the asparagine bound to the beads, 2) there are self-self interactions formed between the antibodies, reducing the aggregation response upon addition of the beads, 3) bead synthesis failed, 4) taking into account the slight disaggregation in the control experiment (Figure 11), there may have been an aggregation response that simply “canceled out” the disaggregation response, resulting in the appearance of no response. Ultimately, it is likely that all three possible explanations contribute to the lack of response to the addition of BSA-Asparagine beads.
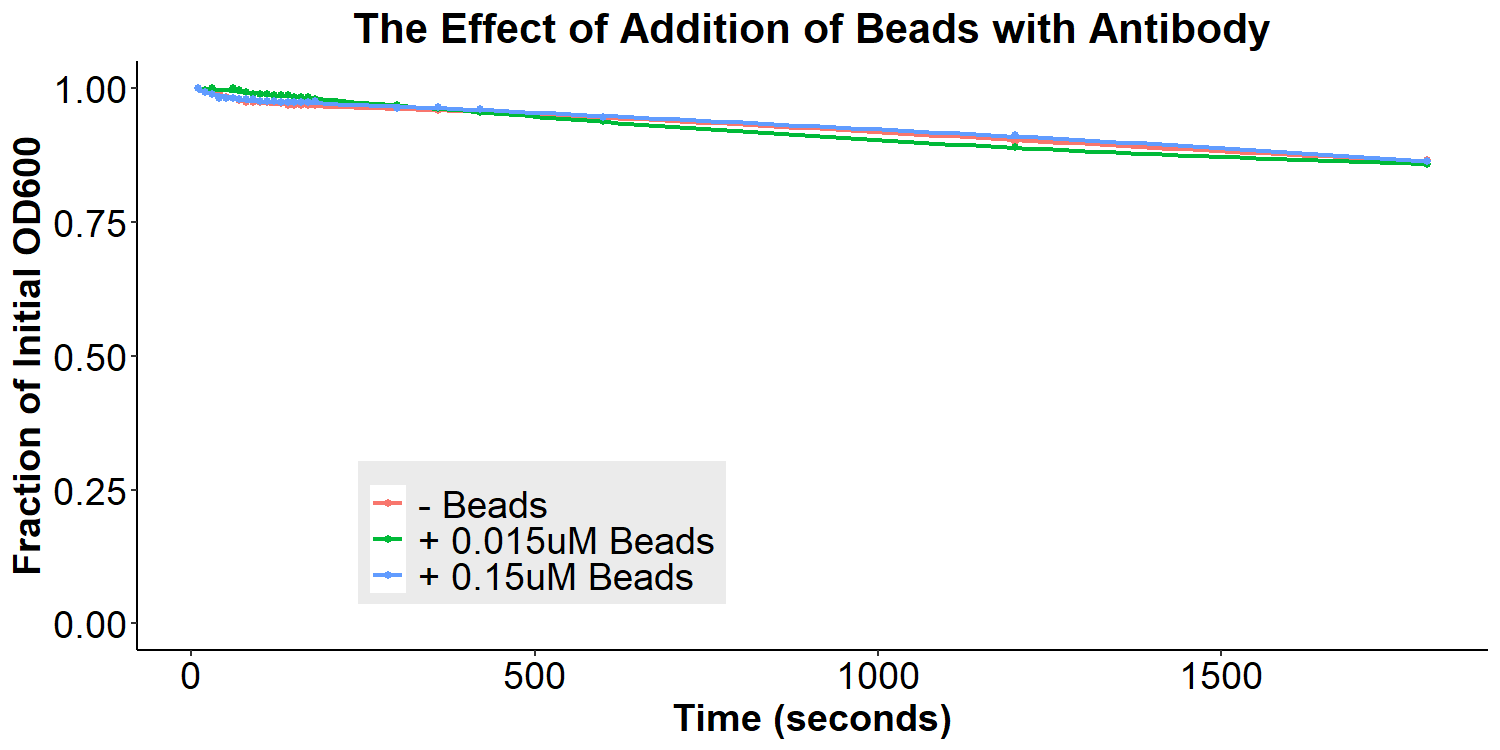
Figure 12: The Effect of Addition of Beads with Antibody-Coated Bacteria Bacterial cultures were first incubated with 2mg/mL antibody for 16 hours. Subsequently, bacterial cultures were incubated in PBS, 0.015uM beads in PBS, or 0.15uM beads in PBS for 6 hours. Bacteria were briefly resuspended and the O.D.600 was measured for 30 minutes.
Biosensor for Asparagine Differentiates Between Asparagine Concentrations
Of greatest importance to the overall project goal is the determination of whether our biosensor can detect the differences between various concentrations of free asparagine. Accordingly, bacteria were incubated with anti-asparagine antibody for 16 hours, washed in PBS, incubated with 0.15uM beads for 6 hours, and incubated with free asparagine (0mM, 30mM, 100mM, or 300mM) for 12 hours (Figure 13). Upon addition of the free asparagine, there is a disaggregation event. This is consistent with the results observed for GFP (Figures 9 and 10). Again, this result is contrary to what was expected with our original model of the biosensor, but is consistent with the new model (Figure 13), explaining that there are interactions between the antibodies, being out-competed upon addition of the antigen.
Importantly, the disaggregation event appears to be asparagine concentration dependent. The 30mM asparagine sample exhibited significantly less disaggregation than the 100mM and 300mM asparagine samples. There was no significant difference between 100mM and 300mM, indicating that these concentrations were out of the range of the biosensor. These experiments suggest that we have successfully created a biosensor for asparagine.
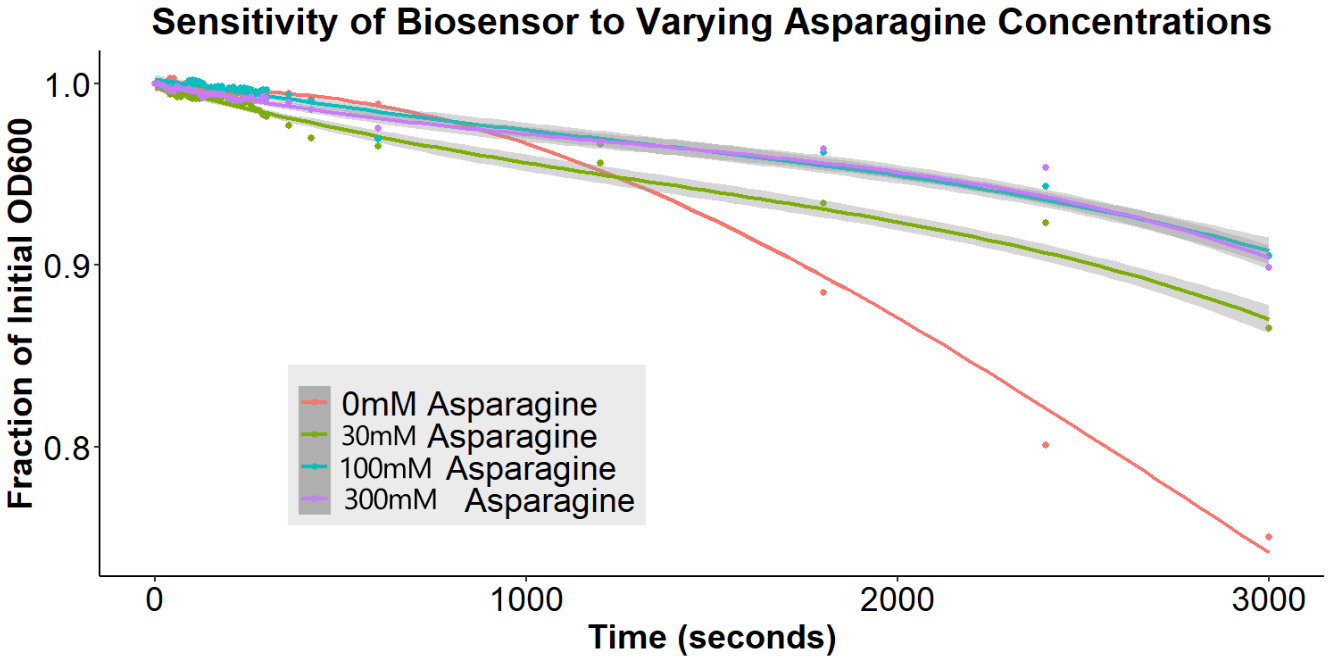
Figure 13: The Effect of Varying Asparagine Concentrations Bacterial cultures were first incubated with 2ug/mL antibody for 16 hours. Subsequently, bacterial cultures were incubated in 1.5uM beads in PBS for 6 hours. Bead-incubated bacteria were then incubated with 0mM, 30mM, 100mM, or 300mM asparagine. Bacteria were briefly resuspended and the O.D.600 was measured for 30 minutes. The gray ribbons represent the 95% confidence interval for the fitted line.
Updated Model of Biosensor Mechanism
Some of the experiments did not support the original hypothesized biosensor model. Specifically, upon addition of antigen, a decrease in aggregation was observed instead of a predicted increase in aggregation. This observation can be observed by our updated model below (Figure 15).
In this new model, addition of the antibody does compete away homophilic EibD-EibD interactions. However, as indicated by the data in Figures 7 and 8, this does not result in zero autoaggregation. This may be because of additional aggregation that is imparted by the antibodies themselves. While the EibD proteins may no longer be interacting, the antibodies may be interacting with each other. These antibody-antibody interactions may not be as strong as the EibD-EibD interactions, causing a reduction, but not full absence, of autoaggregation. In this new model, addition of the antigen, GFP, may therefore cause a decrease in autoaggregation by out-competing the antibody-antibody interactions. This new model may explain the decrease in autoaggregation observed in Figures 9, 10, and 13.

Figure 15: Hypothesized Mechanism of Action of Universal Whole-Cell Biosensor a) uninduced bacteria have relatively few interactions with one another b) upon induction of EibD expression, the bacteria “autoaggregate” together c) addition of the antibody outcompetes most of the EibD homophilic interactions, but also contributes to aggregation due to antibody-antibody interactions d) addition of the antigen outcompetes remaining interactions, reducing the observed autoaggregation
Conclusions
Team Saptasense has made strides toward the development of a novel, antibody-based universal whole-cell bacterial biosensor. When applied to the detection of the protein GFP and the small molecule asparagine, our biosensor was successful in differentiating between different concentrations of the molecules of interest. In further experiments, we hope to apply our new technology to detect other compounds such as biomarkers for disease or environmental hazards. We hope to offer our strain as a “kit” for other scientists to use with their desired antibody, enabling universal detection.
References
[1] Merkel V, Ohder B, Bielaszewska M, Zhang W, Fruth A, Menge C, Borrmann E, Middendorf B, Müthing J, Karch H, Mellmann A. Distribution and phylogeny of immunoglobulin-binding protein G in Shiga toxin-producing Escherichia coli and its association with adherence phenotypes. Infect Immun. 2010 Aug;78(8):3625-36. doi: 10.1128/IAI.00006-10. Epub 2010 Jun 14. PMID: 20547747; PMCID: PMC2916290.
[2] Sandt, C. H., Y. D. Wang, R. A. Wilson, and C. W. Hill. 1997. Escherichia coli strains with nonimmune immunoglobulin-binding activity. Infect. Immun. 65:4572-4579.
[3] Sandt, C. H., and C. W. Hill. 2000. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect. Immun. 68:2205-2214.
[4] Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010 Feb;125(2 Suppl 2):S41-52. doi: 10.1016/j.jaci.2009.09.046. PMID: 20176268; PMCID: PMC3670108.
[5] Leo, J. C., Goldman, A. 2009. The immunoglobulin-binding Eib proteins from Escheria coli are receptors for IgG Fc. Molecular Immunology 46(8-9):1860-1866.
[6] Leo, J. C., Lyskowski, A., Hattula, K., Hartmann, M. D., Schwarz, H., Butcher, S. J., Linke, D., Lupus, A. N., Goldman, A. 2011. The Structure of E. coli IgG-Binding Protein D Suggests a General Model for Bending and Binding in Trimeric Autotransporter Adhesins. Structure 19(7):1021-1030.
[7] D. Linke, T. Riess, I.B. Autenrieth, A. Lupas, V.A. Kempf. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol., 14 (2006), pp. 264-270
[8] Richardson J.S., Richardson D.C. Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc. Natl. Acad. Sci. USA. 2002;99:2754–2759. doi: 10.1073/pnas.052706099.
[9] Wu S.J., Luo J., O’Neil K.T., Kang J., Lacy E.R., Canziani G., Baker A., Huang M., Tang Q.M., Raju T.S., et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng. Des. Sel. 2010;23:643–651. doi: 10.1093/protein/gzq037.
[10] Wang X., Das T.K., Singh S.K., Kumar S. Potential aggregation prone regions in biotherapeutics: A survey of commercial monoclonal antibodies. mAbs. 2009;1:254–267. doi: 10.4161/mabs.1.3.8035.
| None |