Part:BBa_K3791020
Efficient gRNA Ampicillin construct
This composite part contains an efficient gRNA for CRISPRS-Cas12 use and the necessary elements to allow for the proper transcription of the ampicillin efficient gRNA (BBa_K3791015): promoter and terminator. In contrast to the constructs for the transcription of the preliminary gRNAs (not efficient), apart from containing the efficient gRNAs, a terminator was added in order to ensure proper transcription ending. In this way, the gRNA performance improves, thus obtaining better results with this construct when compared to the not efficient (initial) ones.
The promoter used was T7 (BBa_K1614000), which is a strong promoter that allows transcribing a high amount of the gene of interest in a short period of time. When it is used in pertinent competent cells, it is indirectly inducible by the addition of IPTG (as the T7 RNA-polymerase needs IPTG to be expressed) and therefore permits transcriptional modulation. Finally, the terminator chosen was L3S2P21 (BBa_K2675031).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 81
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
1. Usage and biology: reporter and fluorescence
This part is used inside BL21 competent cells, where we cotransform a plasmid containing the current part, and another containing the LbCas12a (BBa_K2927005) protein. To build our plasmids we used Golden Gate assemby, and to transform them into BL21 cells we used Heat Shock transformation. More details about the building process are available on our Biosensor library building page.
In order to determine if our sensors worked properly we had to first induce our plasmids expression using IPTG (100uM) and then lysate the cells for liberating our CRISPR-Cas construct. The method used for that was enzymatic lysis (using a buffer without EDTA). After lysing our cells we proceed with the detection.
As context we must point out that for our CRISPR-Cas ensemble to be a detection system, what we did was to add a reporter to the media where the Cas may become active. The basis relies on LbCas12a’s special property of collateral trans cleavage activity. That means that when it recognizes the specific target sequence determined by the designed gRNA with which it is coupled (thus becoming active) it unspecifically cuts not only the target DNA but also any dsDNA present in the media. In this way, whenever Cas is activated, fluorescence arises because the reporter is cut and releases the fluorescent molecule. All this is step-by-step indicated in Figure 1.
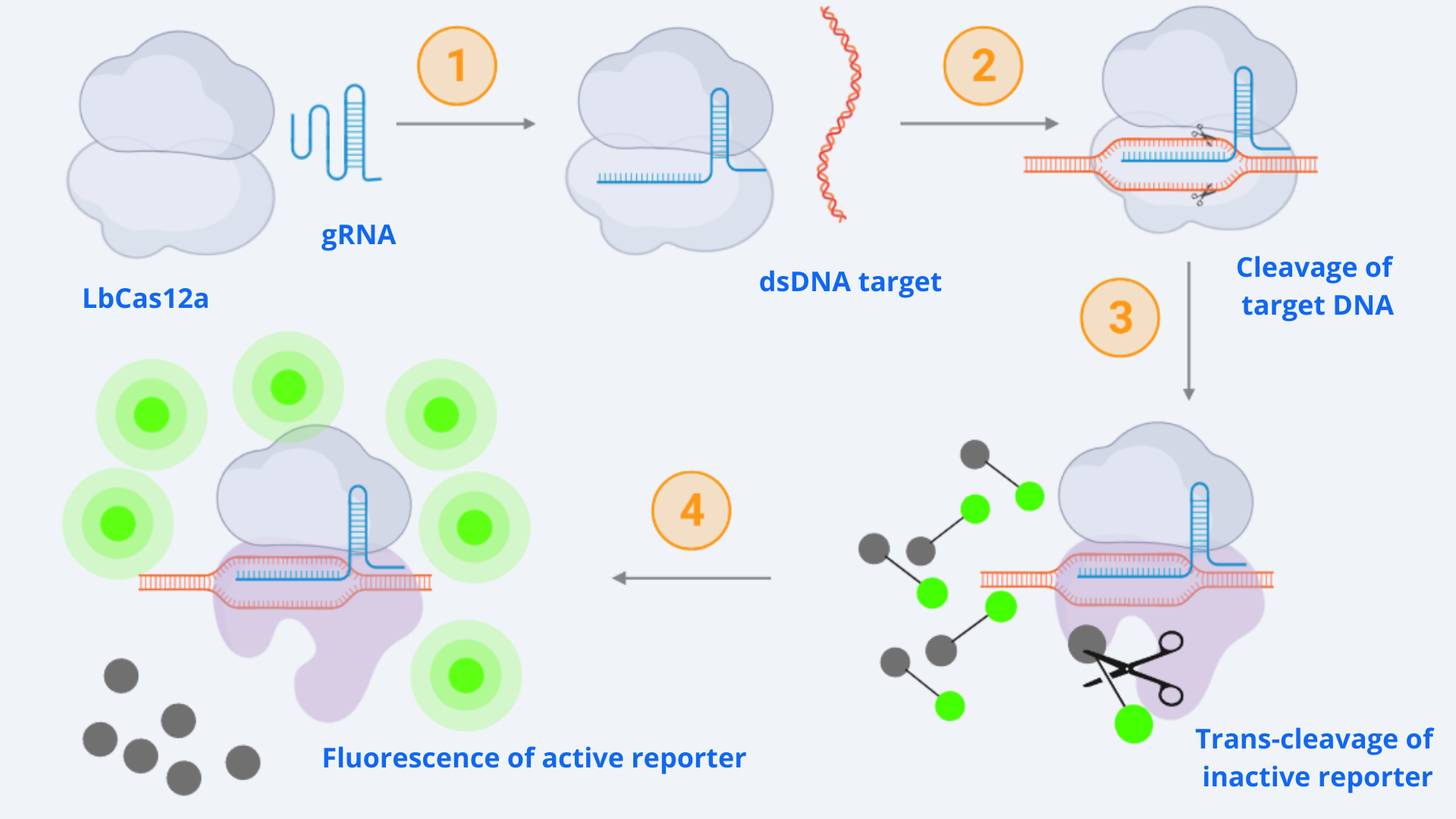
Figure 1 Description: General schematic of Cas12-gRNA-reporter functioning containing the main enzymatic reactions. Cas12 binds to the gRNA, this complex gets activated by recognizing the specific target and cis-cleavage activity starts by cutting this dsDNA target. After that, cas12 trans-cleavage collateral activity cuts any nonspecific DNA fragment, cleaving the reporter. The reporter contains a quencher, which inhibits the fluorophore, and when cutted, luminescence comes out.
2. Characterization: model
CRISPR-Cas12 enzyme kinetics model was created by ARIA with the aim of understanding the dynamics and limitations of using the CRISPR-Cas technology for detection purposes. The model is extensively explained in our more complete composite part: BBa_K3791021, since the experimental parameters and results used for comparing with simulations were the ones performed with this part.
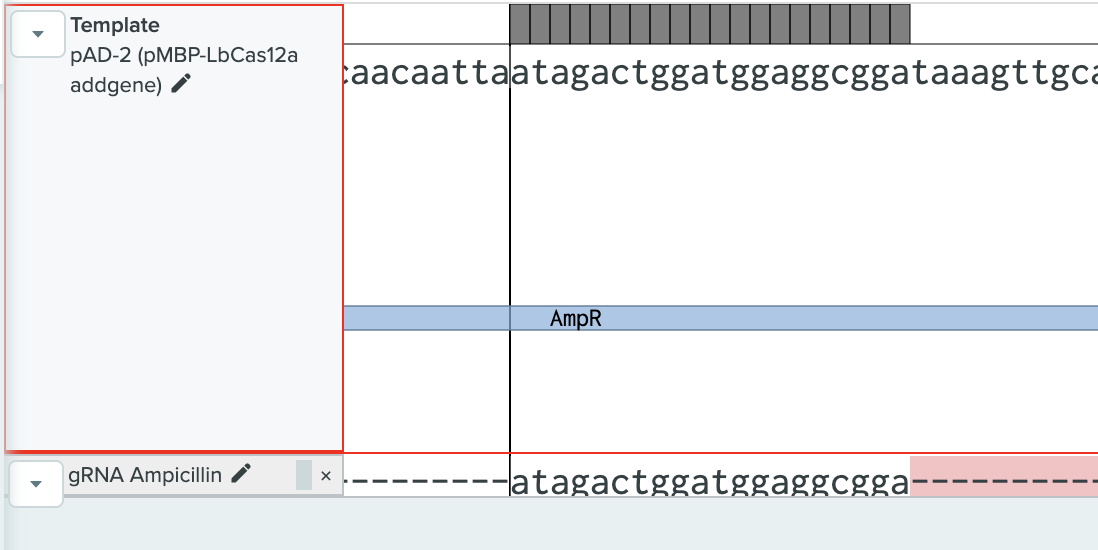
We did not perform any experiments with the gRNA Ampicillin construct (BBa_K3791010) nor Efficient gRNA Ampicillin construct (BBa_K3791020), because of an impairment of its design. It was observed that colonies and cultures for these constructs were not viable. This led to a review of the design of these particular parts. It was hypothesized that the assembled gRNA-Cas12a cleaving was targeting the plasmid’s selection resistance gene, in this case, Ampicillin (see Figure 2). Cleavage of this particular sequence made the resistance gene unfunctional, provoking the cell’s death when cultured with the aforementioned antibiotic. This event actually proved that the gRNA-Cas12a was functional and able to cleave the targeted sequence, which is an important proof of our system viability. Our error in the gRNA design allowed us to verify biosensors functioning.
//function/crispr/grna/construct
//function/crispr/grna/efficient
| None |