Part:BBa_K3512042
Atmosphere Regulated Killswitch
Advances in synthetic biology raise increasingly essential questions about biocontamination and biological containment. It becomes imperative that genetically-engineered organisms are not deleterious to the ecosystem and that scientists have adequate control over their production and multiplication.
We intend to introduce our strain of bacteria into the sugarcane matrix to synthesise anti-invertase. Considering the close proximity of the system with both agricultural environments and human consumables, it is indispensable that our bacteria include a lethality mechanism. Certain safeguards are developed to ensure all possible safety — which are then introduced into the genetically modified organism — that compels the organism, in our case the bacteria, to rely on a “survival” factor to maintain viability in the population. Kill switches when introduced into the genetic material results in cellular death under certain conditions, such as the activation of a “trigger” caused by environmental changes. Thus helping us effectively control the proliferation of the genetically-engineered microorganism.
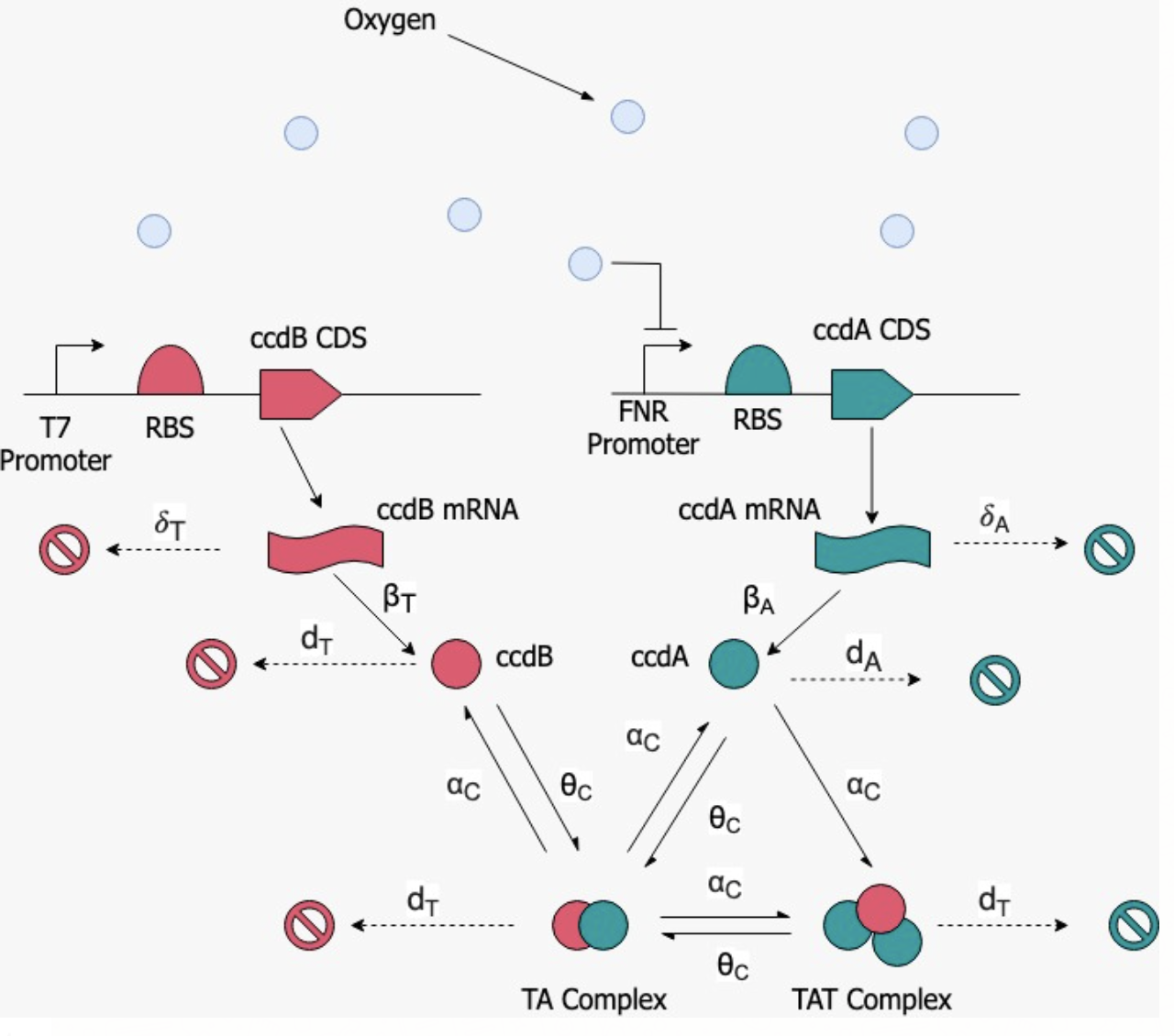
We designed a modified type II ccdA-ccdB toxin-antitoxin system as our kill switch that is activated upon exposure to atmospheric concentrations of oxygen. A change in the oxygen concentration in the environment results in a sharp increase in the amount of the toxin protein being synthesised which disturbs the already established equilibrium between the toxin and antitoxin, effectively killing the cell, and thus preventing any contamination by stray microbes. The CcdB toxin is also harmless to human cells making it safe to consume.
Type II Toxin-Antitoxin Systems
Bacterial toxin-antitoxin systems were characterised in the 1980s as molecular systems encoded in plasmids that ensured the persistence of a plasmid in the host lineage during replication by making the cells “addicted” to the plasmid so that the only daughter cells that survived were the ones that contained the plasmid. This phenomenon is also known as post-segregational killing. Most prokaryotic chromosomes contain a number of toxin-antitoxin modules that consist of a pair of genes that encode for two components: a stable toxin protein and its unstable, labile cognate antitoxin. Toxins are always proteins, but the antitoxins can either be RNAs (in type I and III systems) or proteins (in type II systems) (Syed, M. & Lévesque, C., 2012).
Type II toxin-antitoxin systems use a protein antitoxin to deactivate the toxin protein via protein-protein interaction. All type II antitoxins are dual-function, two-domain proteins that consist of a protein-protein interaction domain and a DNA-binding domain. The antitoxin binds to the toxin and inhibits its activity, and the stable TA complex binds to the operator of the corresponding TAS operon via the DNA-binding domain of the antitoxin and represses its transcription (Makarova et al., 2009) (Tam & Kline, 1989).
Features
ccdA-ccdB System
In the ccdA-ccdB system, the two genes are organised in an operon in which the antitoxin ccdA gene is upstream of the toxin ccdB gene. ccdA prevents ccdB toxicity by forming a tight ccdA-ccdB complex (Afif et al., 2001). ccdB inhibits DNA replication by poisoning DNA gyrase; more specifically, ccdB prevents DNA gyrase from disentangling DNA from replication resulting in double-stranded breaks in the DNA (Dao-Thi et al., 2005). ccdA and ccdB are able to form complexes of various stoichiometries depending on their molar ratios.
Our kill switch is a modified version of the ccdA-ccdB TA system (BBa_K3512001, BBa_K3512002), where the ccdB (toxin) CDS is placed under a T7 (BBa_I719005) constitutive promoter, and the ccdA (antitoxin) CDS is placed under an FNR promoter (BBa_K1123000) whose activity is controlled by the concentration of oxygen present in the environment.
FNR Promoter
This part which we used is a standard FNR promoter. The promoter is used in E. coli strains to induce protein expression in anaerobic environments. It is natively used to induce metabolic processes when the E. coli enter anaerobic environments. The part is often used to express any protein sequence placed behind it in such environmental conditions. On entering a hypoxic environment, the formation of [4Fe−4S]2+ dimers is stimulated within the FNR-producing bacteria. These dimers are the transcription activators of the FNR promoter. They are able to bind to the promoter, thus allowing it to become active. Important to note is that the biobrick (number) is a tandem promoter meaning that two [4Fe−4S]2+ dimers are able to bind to the promoter. This tandem effect enhances the induction effect of the promoter, ultimately leading to a higher expression rate. As the environment inside sugarcane is hypoxic compared to the external atmosphere, we chose to place the ccdA antitoxin under the FNR promoter.
Mechanism
The kill switch function is best explained with the help of two different cases: the first where the bacteria is present in a sufficiently oxygenated environment, and the second where it is present in a hypoxic one.
Hypoxic Environment
The ccdB toxin is under a constitutive promoter which constantly expresses the toxin inside the cell. Due to prevalant hypoxic conditions inside the sugarcane (Bieleski, R. L., 1960) conditions, the FNR promoter will be active and hence the ccdA antitoxin will also be produced in the cell. Since both ccdA and ccdB are present in the cell, they form a highly stable toxin-antitoxin complex. This way the bacteria is able to survive inside the sugarcane stem.
Aerobic Environment
In this case, too, ccdB is expressed constitutively with the help of the T7 promoter; however, due to the presence of oxygen, the FNR promoter is switched off. This leads to an increase in the levels of ccdB in the cell which in turn leads to a complete halt in cell division and death. This ensures that the bacteria will not be able to survive outside the sugarcane environment.
Modelling
Six different chemical species have to be accounted for: the toxin mRNA (MT), the antitoxin mRNA (MA), the toxin (T), the antitoxin (A), a complex of the form TA (C1), and another complex of the form TAT (C2); the concentrations of these species are determined by forming ordinary differential equations.
We make the following assumptions in our model:
Our inducible mechanisms follow Hill characteristics.
a.(Quasi-steady state approximation) Due to a difference in the time scales involved between binding/unbinding of FNR and oxygen (happening in the order of milliseconds) and transcription/translation (happening in the order of minutes), we assume that the concentration of the oxidised FNR promoter, [FNR−O2] remains time-invariant, that is, d[FNR−O2]dt=0.
b. The transcription rates for both the toxin and antitoxin mRNAs were assumed to be approximately similar.
c. The initial concentrations of all chemical species was assumed to be zero.
Differential Equations
We write the coupled ODEs that describe the reactions illustrated above as follows.
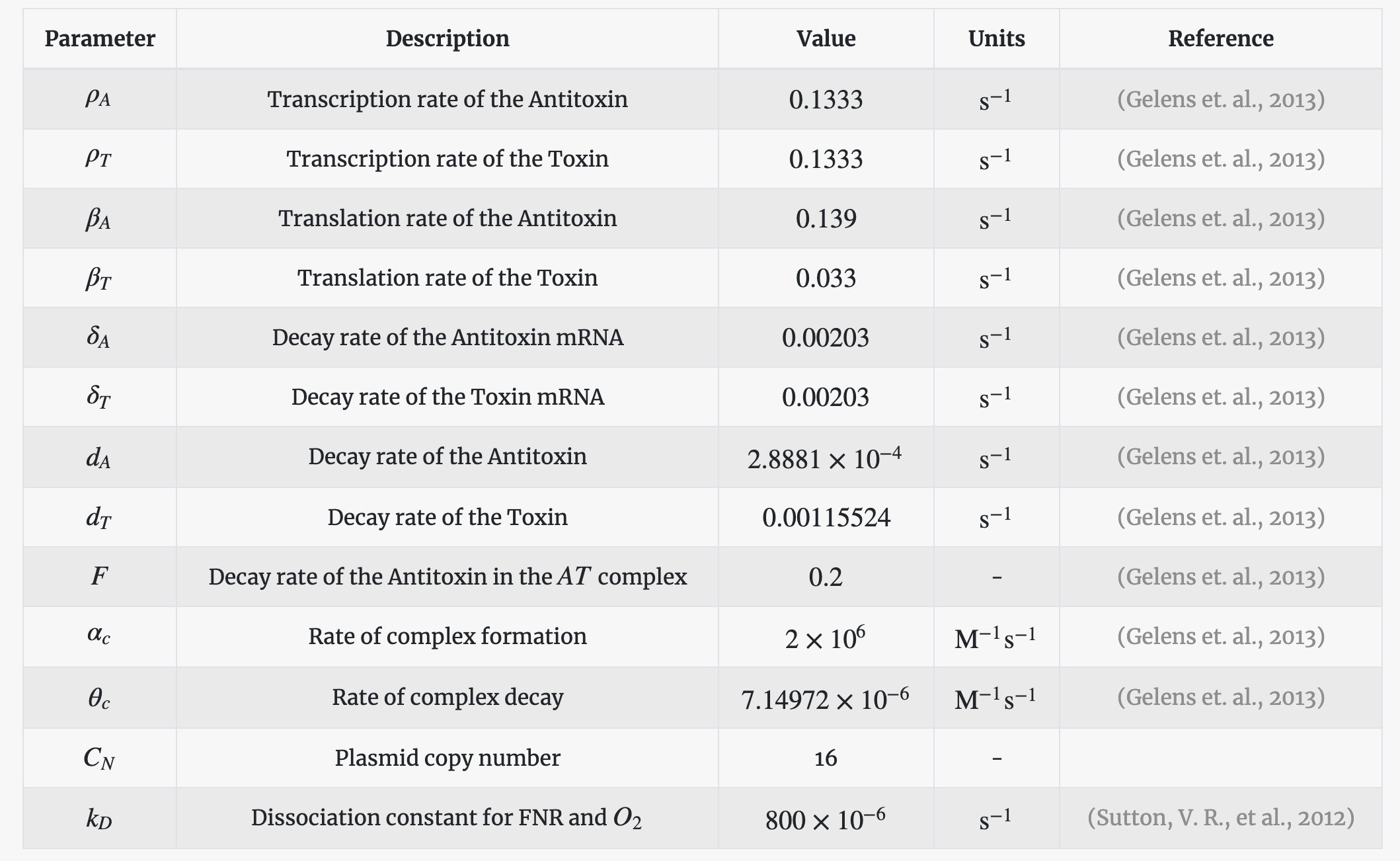
The parameters used in the above set of equations along with their description and numerical values are tabulated below. In the absence of literature on the FNR/O2 interactions, we have made educated guessed about some parameter values.
Parameters
Simulation of Model
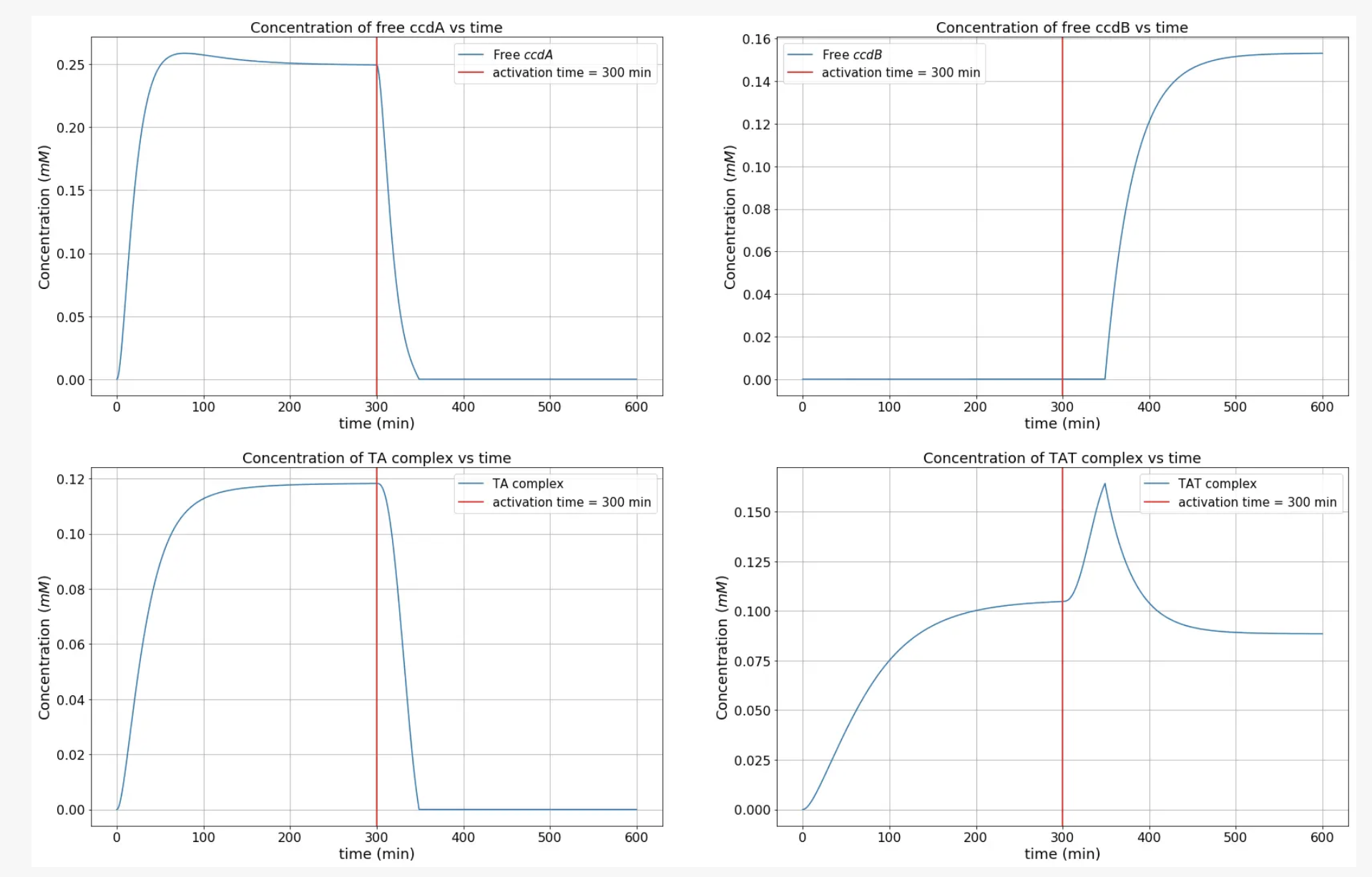
From the graph, it is seen that around the t=300 min mark (when the kill switch is activated), the concentration of free ccdA begins to drop, reaches a minimum and that of free ccdB beings to rise. After a while, the only major chemical species in the cellular system are the toxin (ccdB) and a highly stable TAT complexe. Therefore, the toxin accumulates in the cell and reaches a high enough concentration to kill it.
Sensitivity Analysis
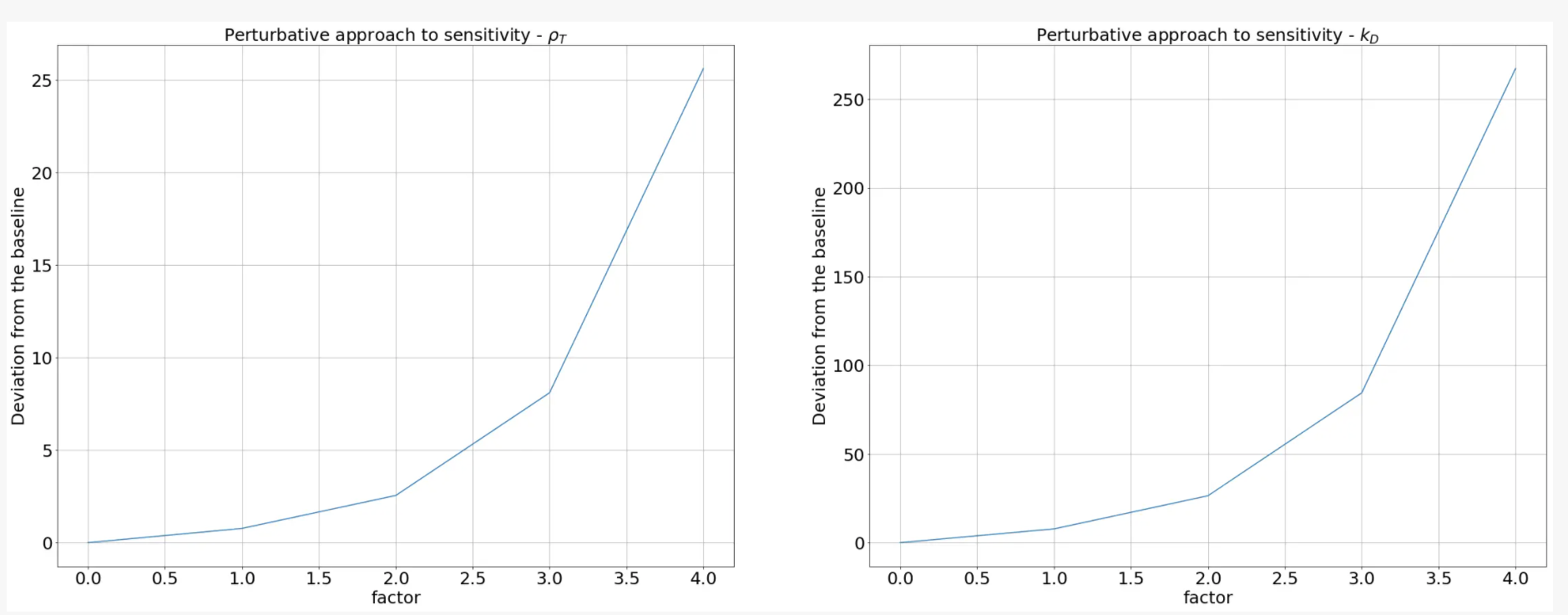
The results of our sensitivity analysis showed that manipulating parameters associated with the FNR promoter would result in a more significant diference in the model output. Therefore, the amount of toxin produced, and consequently the growth rate of the bacteria can be controlled more easily by varying the kD associated with the FNR promoter. Based on these results, we concluded that somehow altering kD would lead to a more fine-tuned kill switch.
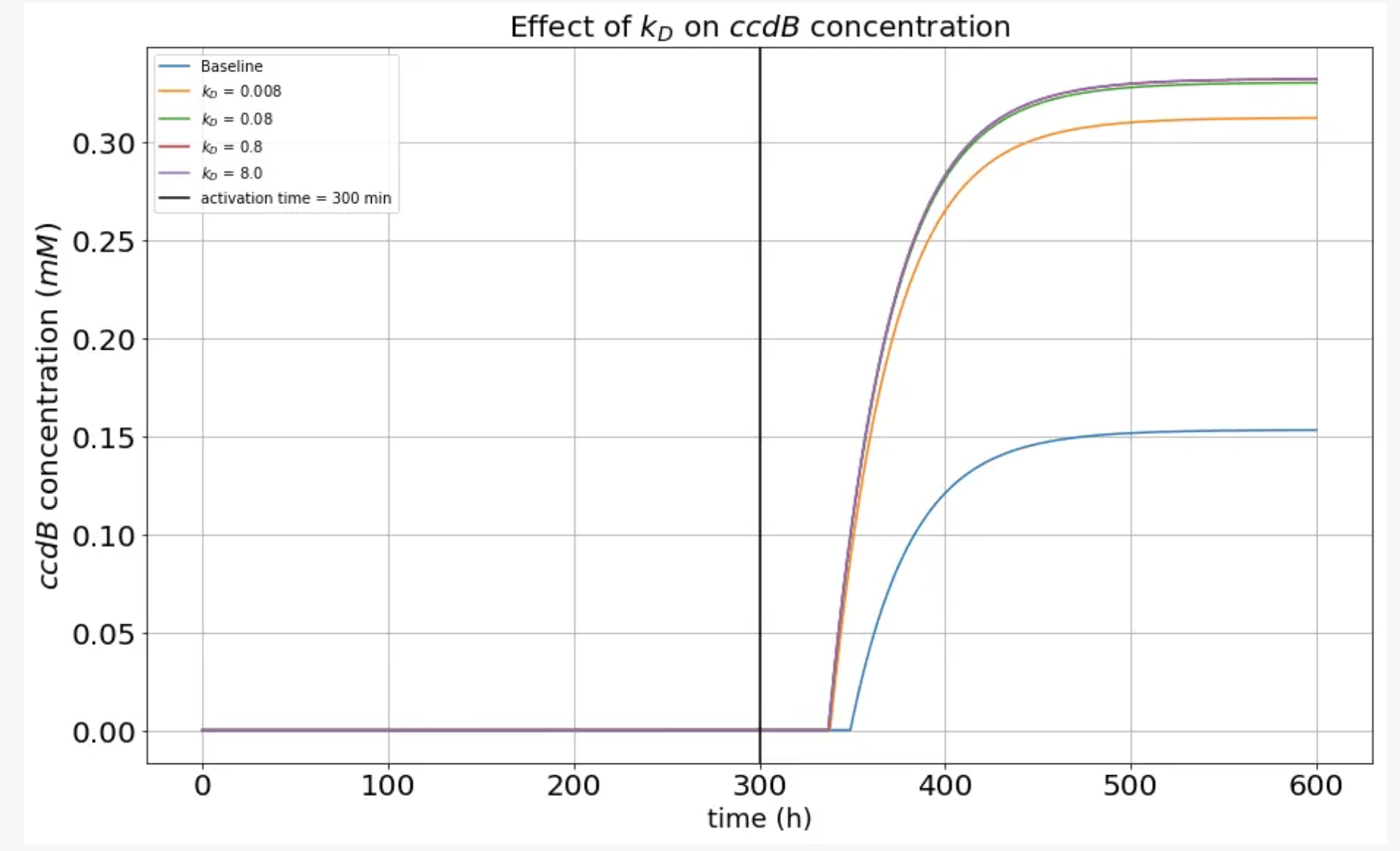
Next we wanted to exclusively study the the variation of toxin concentration as a function of the kD values. To do this, we used the same approach as mentioned above, where multiplied a term proportional to a factor with kD value and studied how the concentration of ccdB would change. The plot we obtained is illustrated below.
This analysis showed us that altering kD is an effective tool to increase the sensitivity of the kill switch as the graph indicates shorter response times for a perturbed kD
Conclusion
From the model we created, we have derived the following conclusions:
a. The arrest of growth can be observed around 50-70 minutes after the kill switch has been activated. b. This time can be brought down further by tuning the model to change the kD value for FNR/O2.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 39
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 739
References
- Syed, M. A., & L vesque, C. M. (2012). Chromosomal bacterial type II toxin-antitoxin systems. Canadian Journal of Microbiology 58(5), 553-562.
- Makarova, K. S., Wolf, Y. I., & Koonin, E. V. (2009). Comprehensive comparative-genomic analysis of Type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biology Direct 4(1), 19.
- Tam, J. E., & Kline, B. C. (1989). The F plasmid ccd autorepressor is a complex of CcdA and CcdB proteins. Molecular and General Genetics MGG 219(1-2), 26-32.
- Reschner, A., Scohy, S., Vandermeulen, G., Daukandt, M., Jacques, C., Michel, B., Nauwynck, H., Xhonneux, F., Préat, V., Vanderplasschen, A. & Szpirer, C. (2013). Use of Staby® technology for development and production of DNA vaccines free of antibiotic resistance gene. Human Vaccines & Immunotherapeutics 9(10), 2203-2210.
- Afif, H., Allali, N., Couturier, M., & Van Melderen, L. (2001). The ratio between CcdA and CcdB modulates the transcriptional repression of the ccd poison-antidote system. Molecular Microbiology 41(1), 73-82.
- Dao-Thi, M.-H., Van Melderen, L., De Genst, E., Afif, H., Buts, L., Wyns, L., & Loris, R. (2005). Molecular Basis of Gyrase Poisoning by the Addiction Toxin CcdB. Journal of Molecular Biology 348(5), 1091-1102.
| None |