Part:BBa_K3264019
NT-2Rep-CT-sfGFP
NT-2Rep-CT-sfGFP is the recombinant spider silk protein (spidroin) that can spin into artificial spider silk fiber. This part is in the part collection where we provide improved recombinant chromoproteins and spidroins based on the functions of different regions of naturally occurring spidroin. By carrying out different mixing of the proteins of different combinations, we can produce artificially spun silk of different colors and properties.
The part collection includes: Parts that are different kinds of spidroins for silk spining. BBa_K3264000 BBa_K3264001 BBa_K3264002 BBa_K3264003 BBa_K3264004 BBa_K3264005 BBa_K3264007 BBa_K3264009 BBa_K3264018. BBa_K3264019. Parts that for combining with the spidroins to form color silks BBa_K3264012. BBa_K3264013. BBa_K3264014. BBa_K3264015. BBa_K3264016. BBa_K3264017. Part for adding more extensive repetitive region to the spidroin protein BBa_K3264011.
Our part collection can help and inspire future teams to further design recombinant spider silks of more variety and give silks diversified color and property to fulfill the collection.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 1060
Usage and Biology
sfGFP-NT-Rep-CT is the spidroin that can be artificially spun into the continuous and high function spider silk with green fluorescence property. This year, we synthesized the part NT-2Rep-CT, to for artificial silk spinning, is the common architecture in two main types of spider silk proteins: major ambulate spidorins(MaSps) and minor ampullate spidorins (MiSps): A non-repetitive N-terminal domain (NT), as well as the C-terminal domain (CT). In between them is an extensive repetitive region (Rep). We believe this part can realize the approach of producing recombinant spidroins (silk proteins) from other chassis and spin them into silk to fulfill the demand of spider silks. However, this part is only responsible for to form continuous silks that are colorless. Inspired by the function NT-2Rep-CT spidroin and in order to reach a larger range of functions, and in order to apply the artificial spun silk to a wider range of industries such as the clothes industry. We first applied the simplest approach by adding super folder GFP(sfGFP) directly into the sequence of NT2RepCT to obtain two structures: sfGFP-NT2RepCT and NT2RepCT-sfGFP. The two kinds of improvements on the original NT-2Rep-CT can give the silk spun different color, but the silk spun by eat of them also vary with each other.
Reference
[1] Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269. [2] Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269.
Characterization
We aimed to use a synthetic biology approach to combine standardized DNA part sfGFP-NT-2Rep-CT, and transform constructs to metabolically engineered Escherichia col (E.coli) for bioproduction.
Fiber spinning
After non-denaturing protein purification, we confirmed their molecular mass of kDa. Silk generated through isopropanol demonstrates certain variability due to the terminal connected to chromoprotein.
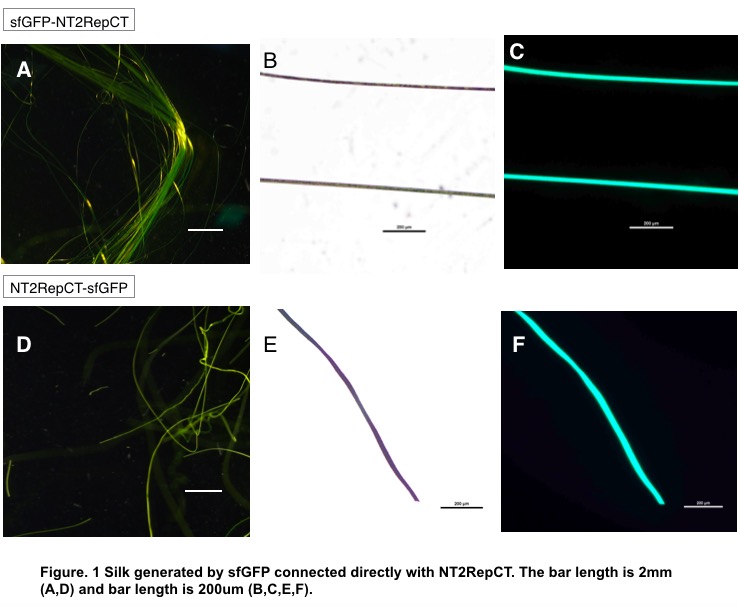
When connected to NT, that is, sfGFP-NT2RepCT, the silk is continuous and relatively transparent (Fig.1.A). Under ultraviolet rays, the silk revealed fluorescent green as expected, and even held certain golden luster under stereoscope with white light.
When connected to CT, however, the silk, with a larger diameter and less transparent (Fig.1.F), is fragile and discontinuous, making it hard to be formed in coagulation bath. The addition of chromoprotein is therefore hypothesized to interfere with CT’s function.
We then tried to apply the same method on eforRed. Unexpectedly, we discovered that spinning dope cannot be extracted through non-denaturing protein purification. The experience with eforRed proved that this manner is subjected to a high risk of silk formation failure and cannot help us to achieve a well-applicable model of colored spider silk.
| None |