Difference between revisions of "Part:BBa K3182103"
| Line 50: | Line 50: | ||
<h1>Usage and Biology</h1> | <h1>Usage and Biology</h1> | ||
| − | <h2> | + | The desired function of this construct is that the CBD will allow a more efficient expression of antimicrobial peptides by being a hydrophilic anchor to the bactericidal peptide. The CBD can be used for easy purification of the fusion protein and via thrombin cleavage of the linker yield the peptide only. In Linköping iGEM's project the thought is to create an antimicrobial bandage by having a polysaccharide bandage with CBD-antimicrobial agent bound to it. When applying the bandage to a patient, the patient's blood will release the peptide into the wound (thrombin sprays used in hospitals can be used as well) leading to the peptide being activated and reaching bacteria, therefore eliminating them. |
| − | + | <h2>Expression, purification and protease treatment</h2> | |
| − | + | The antimicrobial agents which iGEM Linköping used were all expressed the same way. Early expression tests showed great promise but with low concentrations. The early tests were done with standard E. coli expression, cultures were grown to an OD600 of 0.4-1.0 in varying volumes and induced with 0.5 mM IPTG, and let to express the agents for 16 hours in 18 degrees Celsius. Harvest of the cells was done at 3500 rpm for 30 minutes. | |
| − | + | ||
| + | <h3>Solubization and purification</h3> | ||
| + | |||
| + | [[File:T--Linkoping Sweden--SDSLUL.png|300px|thumb|left|<b>Figure B</b> (A) CBD-Pln1 purified and cleaved with thrombin run on an SDS-PAGE 4-20 % gel. Lane 1 contains CBD-Pln1 previously bound to cellulose and treated with thrombin, which explains the multiple bands. Lane 2 contains the cleaved Pln1 from lane 1, which was found in the supernatant from the cleavage along with thrombin (not visible). Lane 3 contains a protein ladder called LMW by GE Healthcare. (B) CBD-Pln1 run on an SDS-PAGE 4-20 % gel after purification. Lane 1 contains a protein ladder called Precision Plus Dual color by BioRad. Lane 2 contains purified CBD-Pln1 in fraction 1 after elution with water. Lane 3 contains purified CBD-Pln1 in fraction 2 after elution with water. Lane 4-5 is empty. Lane 6 contains E.coli BL21 (DE3) lysate from induced cells which carries the plasmid expressing CBD-Pln1.]] | ||
| + | |||
| + | <h3>Solubization and purification</h3> | ||
| + | |||
| + | After harvest the cells was re-suspended in phosphate buffered saline (PBS) buffert or CBM-buffert and sonicated for 6 minutes, 30 % amplitude and 30 seconds on, 30 seconds off. The soluble fraction was then purified by attaching the CBD-fusion to cellulose. This showed very feint bands on SDS-PAGE analysis. Because of the low yield from the soluble fraction, a detergent (Triton X100, 1%) was used to re-suspend the lipid-soluble proteins (insoluble fraction). In this fraction a much higher protein concentration could be found. | ||
| + | |||
| + | The Triton-X100 1 % fraction was later bound to microcristallin cellulose by Sigma Aldrich. A culture of 1 L E. coli BL21 (DE3) treated as above and lysed in 60 mL CBM-buffert. Half of that amount was then incubated with 5 g of microcristallin cellulose for 2 hours in 4 degrees Celsius. After binding the CBD-fusion protein to the cellulose, washing was done with either 70 % ethanol, PBS or CBM-buffer. Where 10 mL of each solution was added and incubated with the cellulose for 30 minutes. This step was repeated three times to remove unbound proteins. After washing, elution was done with deionized water, 10 mL was sufficient to elute some CBD-fusion proteins (room temperature), while other needed higher temperatures (37 degrees Celsius). This was done in 50 mL tubes under shaking, in hindsight, a column should be used to better track elution. | ||
| + | |||
| + | <h3>Thrombin treatment</h3> | ||
| + | These bound fractions could also be cleaved with thrombin, where 25 units Bovine Thrombin (100 uL) by Sigma Aldrich was added. To this thrombin cleavage buffert (20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) was also added (500 uL) along with deionized water (4.4 mL). The results from this can be seen in Figure X. | ||
| + | |||
| + | <br><br><br><br><br> | ||
| + | |||
| + | <h2>Antimicrobial activity of bound agents</h2> | ||
| + | |||
| + | [[File:T--Linkoping_Sweden--CBD-pln145.png|310px|thumb|left|<b>Figure B</b> Uncleaved CBD-Pln1 was tested against an E.coli (DE3) 0 OD culture. Unfilled boxes is a negative control containing 160 uL E.coli (DE3) and 40 uL water. The rest of the samples in the graph represent a dilution series of CBD-Pln1 at the same volumes as the negative control. The media used was low salt LB (0.4 g/L NaCl). The error bars represent the mean ± SD from six technical replicates.]] | ||
| + | |||
| + | <h3>CBD bound agents</h3> | ||
| + | |||
| + | An experiment to test the antimicrobial activity of our agents still bound to the CBD was conducted, which can be seen in Figure B. This was done in order to see if the absence of thrombin cleavage would yield inactive agents. The CBD-bound agents were purified like above method mentions. Six technical replicates of E. coli BL21 (DE3)(160 uL in low salt LB-media, 0.4 g/L NaCl) were added to a 96-well plate by Eppendorf. To this 40 uL water was added to function as a negative control (called positive control in figure). To 6x6 wells a concentration gradient was added in the same way, always adding 160 uL (in low salt LB-media) E.coli BL21 (DE3) and 40 uL of the unbound agents in water, diluted to the concentrations in Figure B. The experiment was run for 16 hours in 37 degrees and before each measurement a quick shake (200 rpm, 10 seconds) was done. The absorbance at 600 nm was measured every hour. | ||
| + | |||
| + | <h3>Importance of testing the CBD-bound agents</h3> | ||
| + | |||
| + | High amounts of the CBD-bound agent could still inhibit growth of E. coli, meaning if thrombin is not present in the wound the bandage would still yield a antimicrobial effect, this can further be seen in Figure B (plåstertest). This also explains the trouble when expressing the CBD-agents and why they can be found in the insoluble fraction. | ||
| + | <br><br><br><br><br><br><br><br> | ||
| + | <h2>Antimicrobial activity of unbound agents</h2> | ||
| + | <br> | ||
| + | [[File:T--Linkoping_Sweden--combined.png|600px|thumb|left|<b>Figure B</b><i>B</i>: Cleaved Pln1 was tested against an E. coli BL21 (DE3) 0 OD culture. Unfilled boxes is a negative control containing thrombin and thrombin cleavage buffert with the same volume as Pln1 (40 uL). Filled boxes is Pln1 at a concentration of 3.1 uM <i>C</i>: Pln1 was added to a B. subtilis 0 OD culture. Unfilled boxes indicate negative control containing thrombin and thrombin cleavage buffert with the same volume as Pln1 (40 uL). The media used was low salt LB (0.4 g/L NaCl). The error bars represent the mean ± SD from three independent experiments.]] | ||
| + | <h3>Unbound agents</h3> | ||
| + | As a positive control before starting our experiments with the CBD-agent-bandage, the unbound agents needed to be able to kill bacteria as well. The agents were purified and treated with thrombin as stated before. The experimental setup was the same as the earlier experiment but instead of adding water to the negative control, thrombin (0.5 U, 0.6 uM) and thrombin cleavage buffert (40 uL, 20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) was added. This was because the agents had thrombin and cleavage buffert with them. This showed to not affect the bacterial growth, but rather increase it due to the higher salt amounts in the cleavage buffert. | ||
| + | |||
| + | <br><br><br><br><br> | ||
| + | <h2>Antimicrobial activity immobilized and released</h2> | ||
| + | |||
| + | [[File:T--Linkoping Sweden--pepplåster.png|350px|thumb|left|<b>Figure B.</b> A cellulose bandage (Epiprotect) incubated with E. coli BL21 (DE3) 0 OD cultures. The unfilled boxes indicate a negative control which contain thrombin and thrombin cleavage buffert and cellulose bandage just covering the bottom of the well. The filled boxes is a cellulose bandage which has CBD-Pln1 bound to it, thrombin and thrombin cleavage buffert to release the fusion peptide from the bandage and into solution. The triangles contain a cellulose bandage which has CBD-Pln1 bound to it, thrombin cleavage buffert was added as well to show antimicrobial activity of immobilized CBD-Pln1. The media used was low salt LB (0.4 g/L NaCl). The error bars represent the mean ± SD from two independent experiments.]] | ||
| + | |||
| + | <h3>Immobilization and release on a cellulose bandage</h3> | ||
| + | To show the proof of principle for iGEM19 Linköping's project, the antimicrobial agents were fused to a cellulose bandage (Epiprotect). The cellulose was first incubated with E. coli BL21 (DE3) lysate (20 mL) containing CBD-Pln1 for two hours in 4 degrees Celsius. After immobilization of the agent to cellulose, the bandage was washed three times with CBM-buffert (10 mL), or until no foam can be seen. The foam build-up is because of the lysate also containing 1% Triton X100, which will heavily interfere with the results. After washing, small pieces of bandage was cut out to fit into a well of a 96-well plate. Added to this well was also low salt LB-media (0.4 g/L NaCl) and E. coli BL21 (DE3) at OD600 0. Thrombin cleavage buffert (20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) were added to all wells, and thrombin (0.05 U, 0.6 uM) were added to release the agent into the solution. The negative control had both thrombin cleavage buffert and thrombin. In Figure B. the results from this experiment can be seen. Noticeably, the thrombin released agent inhibited the E. coli growth the most of the combinations tested. This shows that the proof of principle of iGEM19 Linköping's main mechanism is working. | ||
| + | |||
| + | <h3>Thrombin accessibility in wounds</h3> | ||
| + | The thrombin used for releasing the agents into the solution had a concentration of 0.6 uM, this was to mimic the concentration found in blood from wounds. According to ([http://www.ncbi.nlm.nih.gov/pubmed/12871286 K.G Mann et al. 2003]) the concentration of active thrombin during clotting is several hundred nanomolar (nM), depending on the age of the wound. In the experiment above, thrombin concentrations used was 600 nM. This hopefully mimic a real wound as closely as possible, while working in vitro. | ||
Revision as of 06:23, 14 October 2019
Contents
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 580
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Introduction
pT7-CBDcipA-PlyF307-SQ8C
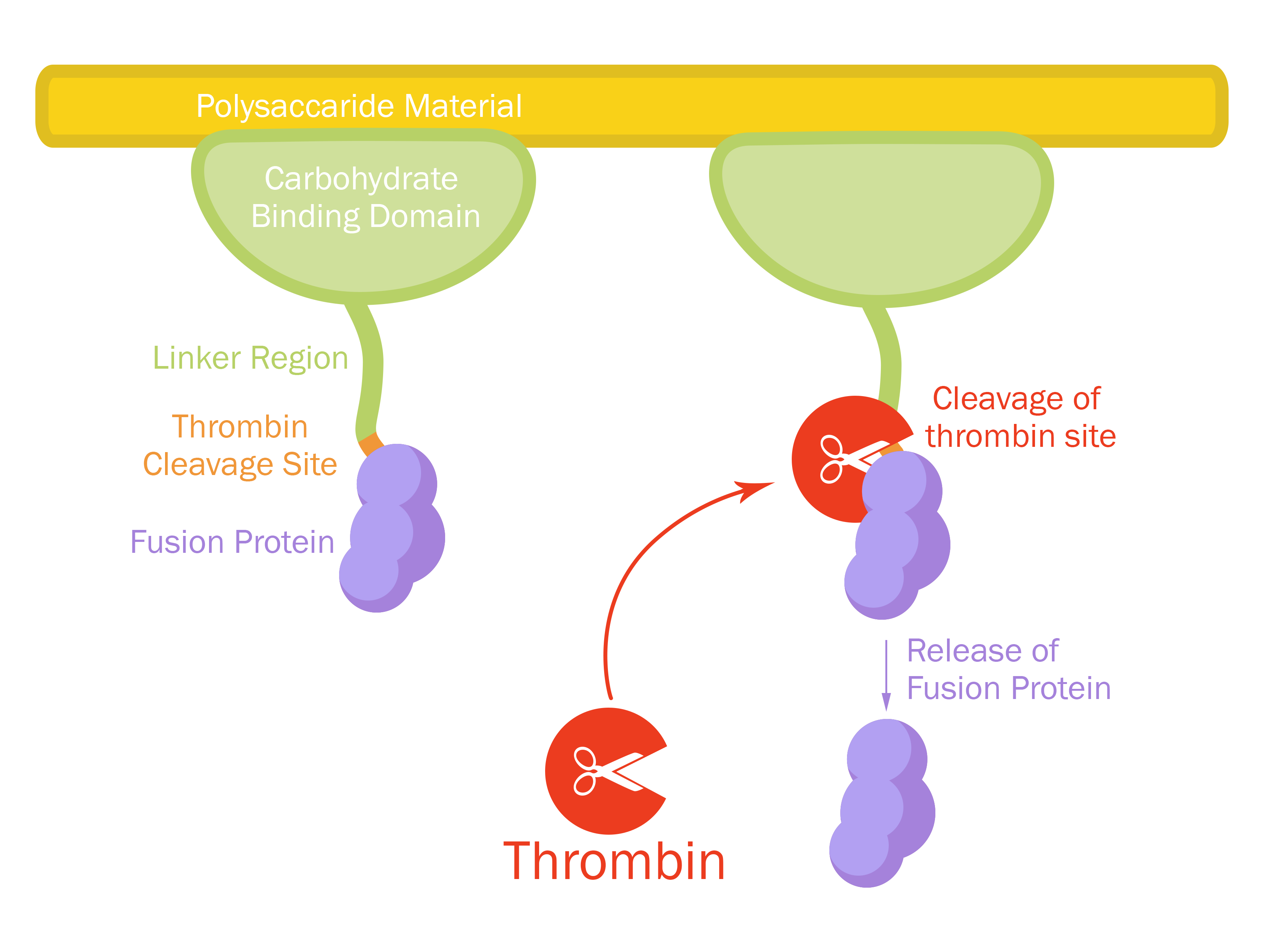
This part consists of a carbohydrate binding domain (CBD) from Clostridium thermocellum (C. thermocellum) cellulose scaffolding protein (CipA). This binding domain is a central part of Clostridium thermocellum's cellusome and has a strong affinity for cellulose. The CBD was fused to another protein using a flexible GS-linker (-GGGGSGGGGS-)in order to attach this complex to a polysaccaride material. A thrombin cleavage site (-LVPRGS-) was added to the end of the linker and its breakage will leave a glycine and serine attached to the N-terminal of the fusion protein.
Protease site and use
The thrombin site was added to enable the ability to release the fusion protein down into skin wounds. Thanks to our integrated human practice we learned that infections span much deeper into wounds that we thought. Simply attaching the CBD-fusion protein to a carbohydrate material would not enable the fusion protein to reach far into the wound. The thrombin site was also chosen because of thrombin's endogenous existence in humans.
Assembly compabilities
An internal BamHI recognition sequence (RS) has been added to enable interchangeable fusion proteins to the CBD. BamHI was chosen because its RS codes for glycine and serine, fitting it to the end of the thrombin site. It is also a cost-effective enzyme and is unaffected by methylated DNA. BamHI is a part of the RFC21 standard.
CBDcipA crystal structure
Important molecular faces
CBDcipA is composed of a nine-stranded beta sandwich with a jelly roll topology and binds a calcium ion. It further contains conserved residues exposed on the surface which map into two clear surfaces on each side of the molecule. One of the faces mainly contains planar strips of aromatic and polar residues which may be the carbohydrate binding part. Further aspects are unknown and unique to this CBD such as the other conserved residues which are contained in a groove.
Carbohydrate binding domain specificity
Since the CBD is from the cellusome of C. thermocellum some research labeled it a cellulose binding domain. However, iGEM19 Linköping noticed that this domain could also bind to different sources of polysaccaride materials. This serves as a domain for iGEM19 Linköpings modular bandage, where the polysaccaride material can be exchanged for other/similar materials and not exclusively cellulose.
The choice of carbohydrate binding domain
iGEM Linköping 2019 chose CBDcipA due to the fact that many other iGEM teams had explored the possibilities of this domain. Our basic design was influenced by [http://2014.igem.org/Team:Imperial iGEM14 Imperial], [http://2015.igem.org/Team:edinburgh iGEM15 Edinburgh] and [http://2018.igem.org/Team:ecuador iGEM18 Ecuador]. Purification and where to place the fusion protein (N- or C-terminal) was determined by studying the former projects. CBDcipA also originates from a thermophilic bacteria which further increases the domain's applications.
Expression system
The part has a very strong expression with a T7-RNA-polymerase promotor (BBa_I719005) as well as a 5'-UTR (BBa_K1758100) region which has been shown to further increase expression in E. coli (BBa_K1758106), ([http://www.ncbi.nlm.nih.gov/pubmed/2676996 Olins et al. 1989]), ([http://www.ncbi.nlm.nih.gov/pubmed/23927491 Takahashi et al. 2013]).

Antimicrobial Agent - PlyF307
The Acineto phage muramidase PlyF307 is fused to the CBD in this part is an endolysin from the Acineto phage, which is classified as a cell-wall hydrolase. Fused to the endolysin is an endogenous antimicrobial peptide and to that peptide, another peptide from the hepatitis B virus, which has been shown to increase activity. The version of this protein were inspired by [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862495/ Thandar et al. 2016].
The endolysin is designed to battle the Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp. family of pathogens (ESKAPE). ESKAPE is a family(ies) of bacteria which has multiple understrains that has evolved resistance to the most commonly used antibiotics.
Usage and Biology
The desired function of this construct is that the CBD will allow a more efficient expression of antimicrobial peptides by being a hydrophilic anchor to the bactericidal peptide. The CBD can be used for easy purification of the fusion protein and via thrombin cleavage of the linker yield the peptide only. In Linköping iGEM's project the thought is to create an antimicrobial bandage by having a polysaccharide bandage with CBD-antimicrobial agent bound to it. When applying the bandage to a patient, the patient's blood will release the peptide into the wound (thrombin sprays used in hospitals can be used as well) leading to the peptide being activated and reaching bacteria, therefore eliminating them.
Expression, purification and protease treatment
The antimicrobial agents which iGEM Linköping used were all expressed the same way. Early expression tests showed great promise but with low concentrations. The early tests were done with standard E. coli expression, cultures were grown to an OD600 of 0.4-1.0 in varying volumes and induced with 0.5 mM IPTG, and let to express the agents for 16 hours in 18 degrees Celsius. Harvest of the cells was done at 3500 rpm for 30 minutes.
Solubization and purification
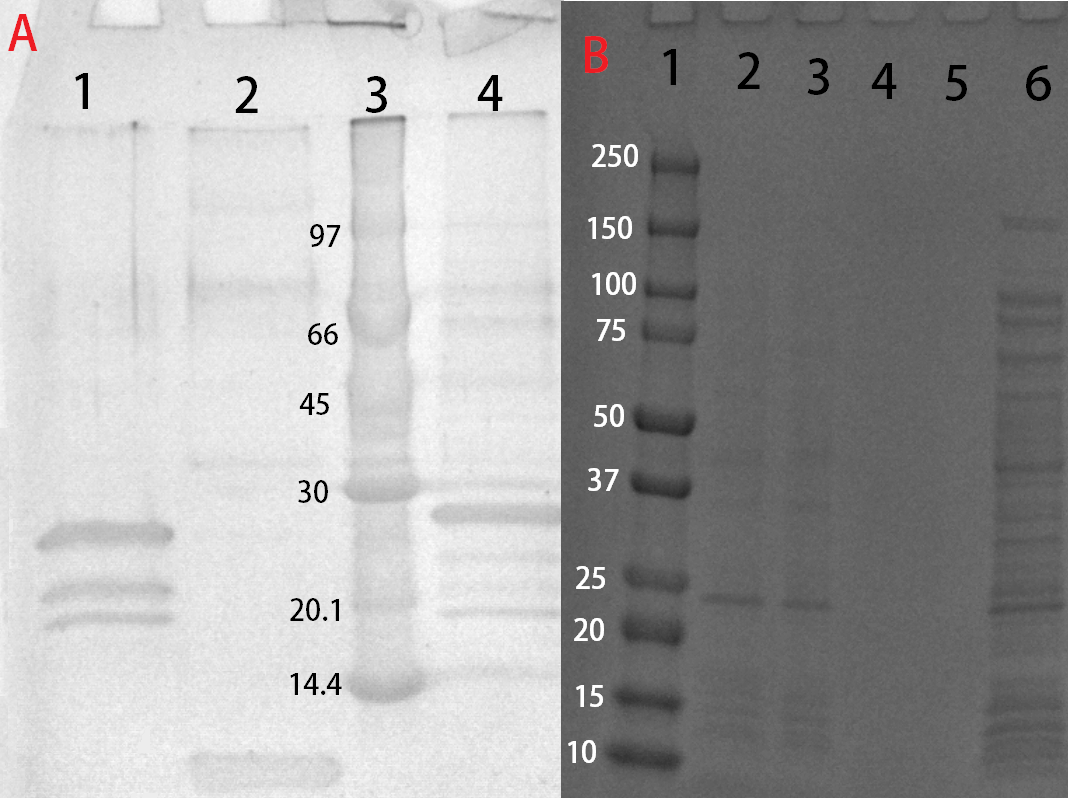
Solubization and purification
After harvest the cells was re-suspended in phosphate buffered saline (PBS) buffert or CBM-buffert and sonicated for 6 minutes, 30 % amplitude and 30 seconds on, 30 seconds off. The soluble fraction was then purified by attaching the CBD-fusion to cellulose. This showed very feint bands on SDS-PAGE analysis. Because of the low yield from the soluble fraction, a detergent (Triton X100, 1%) was used to re-suspend the lipid-soluble proteins (insoluble fraction). In this fraction a much higher protein concentration could be found.
The Triton-X100 1 % fraction was later bound to microcristallin cellulose by Sigma Aldrich. A culture of 1 L E. coli BL21 (DE3) treated as above and lysed in 60 mL CBM-buffert. Half of that amount was then incubated with 5 g of microcristallin cellulose for 2 hours in 4 degrees Celsius. After binding the CBD-fusion protein to the cellulose, washing was done with either 70 % ethanol, PBS or CBM-buffer. Where 10 mL of each solution was added and incubated with the cellulose for 30 minutes. This step was repeated three times to remove unbound proteins. After washing, elution was done with deionized water, 10 mL was sufficient to elute some CBD-fusion proteins (room temperature), while other needed higher temperatures (37 degrees Celsius). This was done in 50 mL tubes under shaking, in hindsight, a column should be used to better track elution.
Thrombin treatment
These bound fractions could also be cleaved with thrombin, where 25 units Bovine Thrombin (100 uL) by Sigma Aldrich was added. To this thrombin cleavage buffert (20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) was also added (500 uL) along with deionized water (4.4 mL). The results from this can be seen in Figure X.
Antimicrobial activity of bound agents
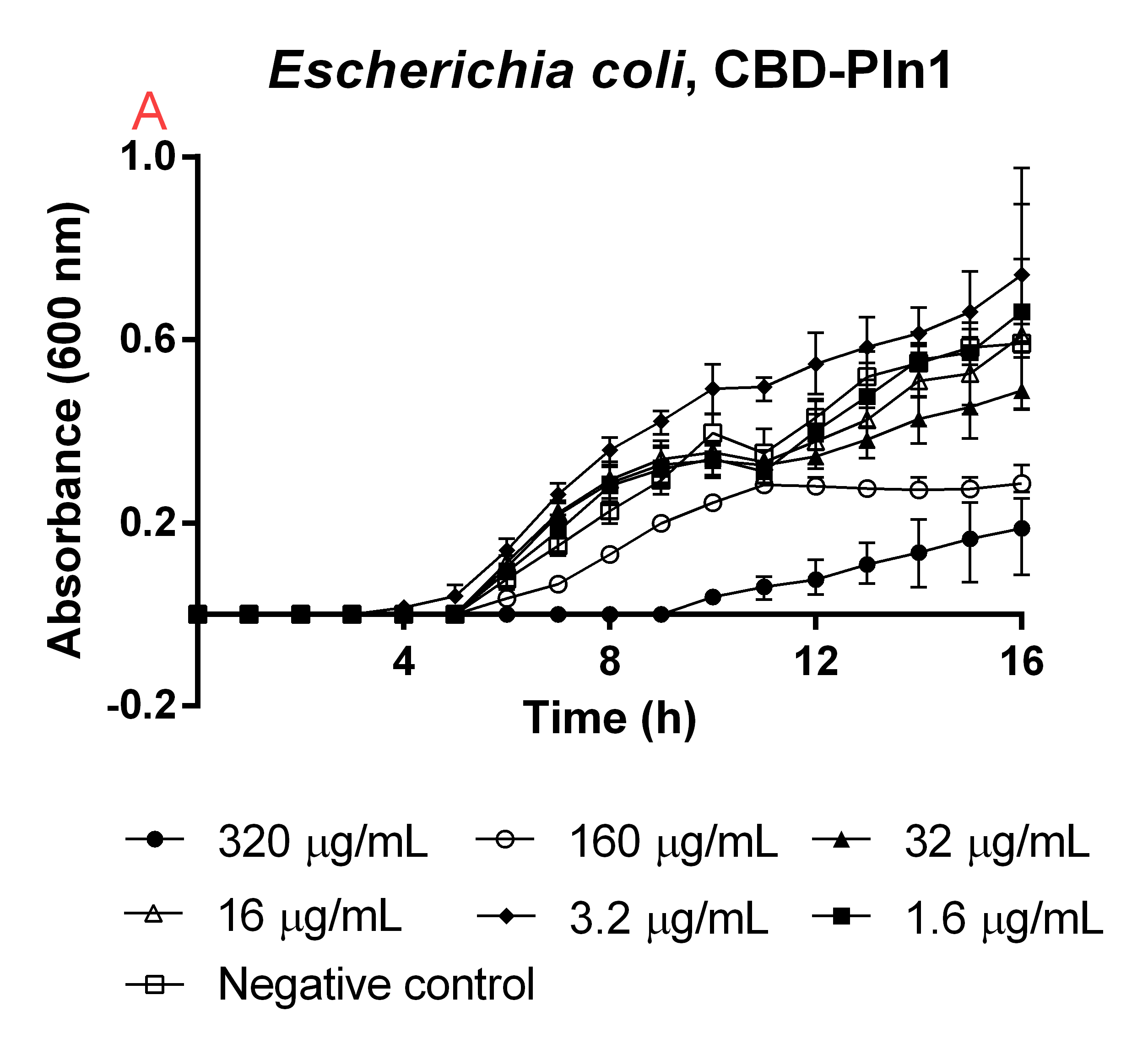
CBD bound agents
An experiment to test the antimicrobial activity of our agents still bound to the CBD was conducted, which can be seen in Figure B. This was done in order to see if the absence of thrombin cleavage would yield inactive agents. The CBD-bound agents were purified like above method mentions. Six technical replicates of E. coli BL21 (DE3)(160 uL in low salt LB-media, 0.4 g/L NaCl) were added to a 96-well plate by Eppendorf. To this 40 uL water was added to function as a negative control (called positive control in figure). To 6x6 wells a concentration gradient was added in the same way, always adding 160 uL (in low salt LB-media) E.coli BL21 (DE3) and 40 uL of the unbound agents in water, diluted to the concentrations in Figure B. The experiment was run for 16 hours in 37 degrees and before each measurement a quick shake (200 rpm, 10 seconds) was done. The absorbance at 600 nm was measured every hour.
Importance of testing the CBD-bound agents
High amounts of the CBD-bound agent could still inhibit growth of E. coli, meaning if thrombin is not present in the wound the bandage would still yield a antimicrobial effect, this can further be seen in Figure B (plåstertest). This also explains the trouble when expressing the CBD-agents and why they can be found in the insoluble fraction.
Antimicrobial activity of unbound agents

Unbound agents
As a positive control before starting our experiments with the CBD-agent-bandage, the unbound agents needed to be able to kill bacteria as well. The agents were purified and treated with thrombin as stated before. The experimental setup was the same as the earlier experiment but instead of adding water to the negative control, thrombin (0.5 U, 0.6 uM) and thrombin cleavage buffert (40 uL, 20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) was added. This was because the agents had thrombin and cleavage buffert with them. This showed to not affect the bacterial growth, but rather increase it due to the higher salt amounts in the cleavage buffert.
Antimicrobial activity immobilized and released
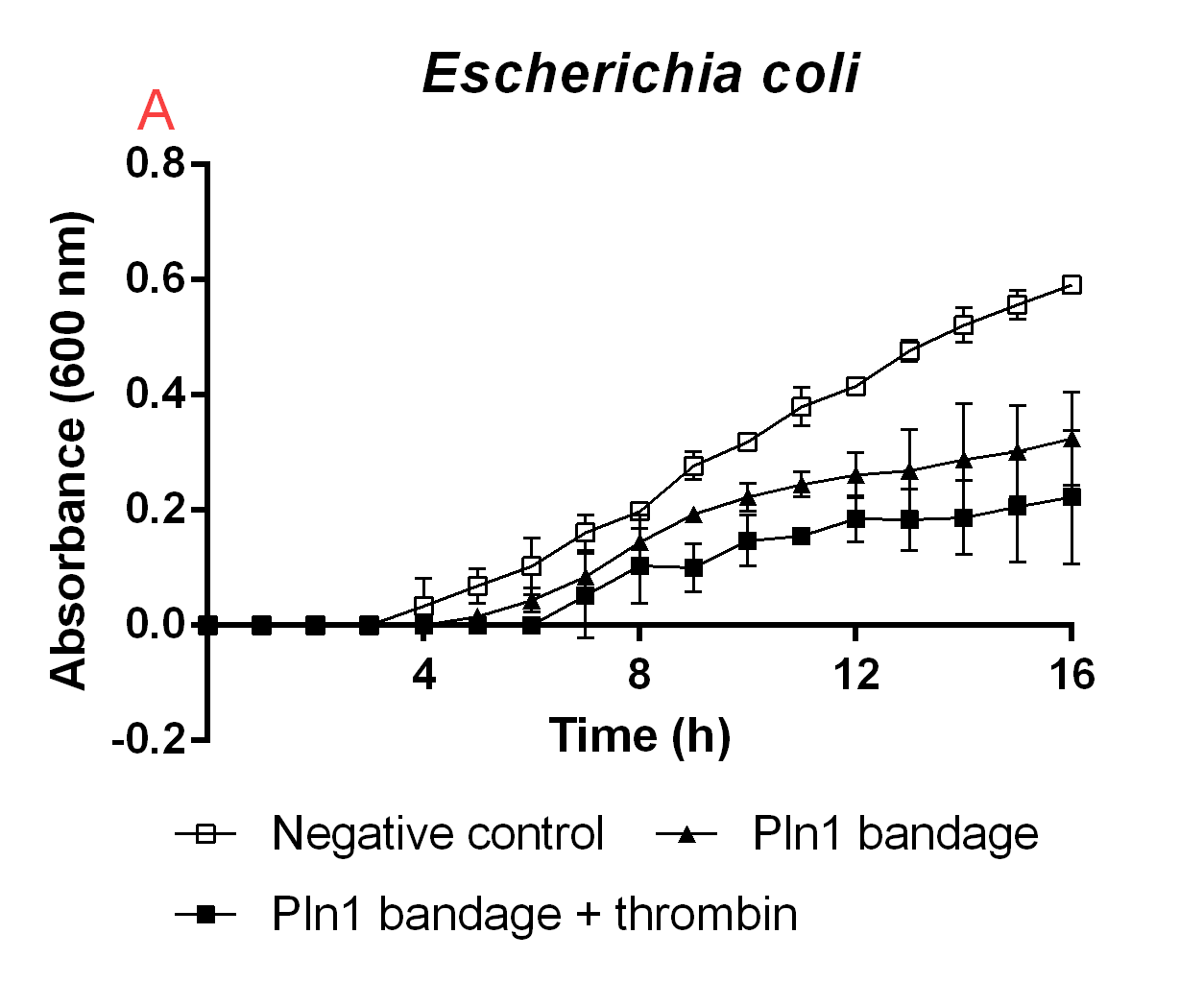
Immobilization and release on a cellulose bandage
To show the proof of principle for iGEM19 Linköping's project, the antimicrobial agents were fused to a cellulose bandage (Epiprotect). The cellulose was first incubated with E. coli BL21 (DE3) lysate (20 mL) containing CBD-Pln1 for two hours in 4 degrees Celsius. After immobilization of the agent to cellulose, the bandage was washed three times with CBM-buffert (10 mL), or until no foam can be seen. The foam build-up is because of the lysate also containing 1% Triton X100, which will heavily interfere with the results. After washing, small pieces of bandage was cut out to fit into a well of a 96-well plate. Added to this well was also low salt LB-media (0.4 g/L NaCl) and E. coli BL21 (DE3) at OD600 0. Thrombin cleavage buffert (20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) were added to all wells, and thrombin (0.05 U, 0.6 uM) were added to release the agent into the solution. The negative control had both thrombin cleavage buffert and thrombin. In Figure B. the results from this experiment can be seen. Noticeably, the thrombin released agent inhibited the E. coli growth the most of the combinations tested. This shows that the proof of principle of iGEM19 Linköping's main mechanism is working.
Thrombin accessibility in wounds
The thrombin used for releasing the agents into the solution had a concentration of 0.6 uM, this was to mimic the concentration found in blood from wounds. According to ([http://www.ncbi.nlm.nih.gov/pubmed/12871286 K.G Mann et al. 2003]) the concentration of active thrombin during clotting is several hundred nanomolar (nM), depending on the age of the wound. In the experiment above, thrombin concentrations used was 600 nM. This hopefully mimic a real wound as closely as possible, while working in vitro.

