Part:BBa_K3052001
(4S)-limonene synthase
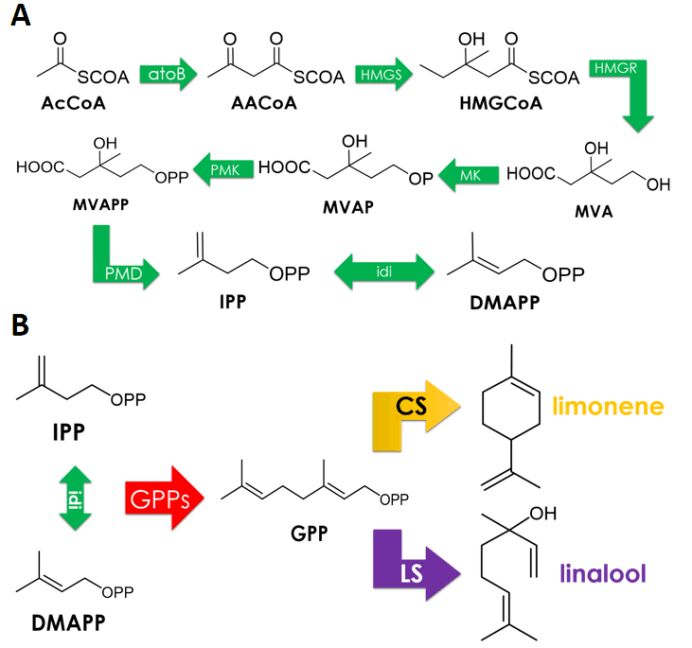
The limonene synthase (CS) (Keasling et al., 2013) sequence used throughout our project which converts GPP to limonene is an E. coli codon-optimized version of a truncated sequence from M. spicata previously described(Hyatt et al., 2007).
Reference [1]lonso-Gutierrez, J., Chan, R., Batth, T. S., Adams, P. D., Keasling, J. D., Petzold, C. J., & Lee, T. S. (2013). Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metabolic engineering, 19, 33-41. Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
Background
In our study, we aim to achieve limonene and linalool synthesis in E.coli DH5α. According to 2018 GreatBay_China team’s experience, no target product was detected using gas chromatography when carrying out shake-flask fermentation with this strain induced by 25uM IPTG for 24 hours due to the lack of endogenous MVA pathways wherein GPP is produced. Thus we decided to co-express an MVA pathway. 2018 GreatBay_China generously gave us one plasmid pJBEI6409(Keasling et al., 2013), which contains a MVA pathway in addition to an GPPs-LS operon. We reconstructed this plasmid and get a plasmid only contains a MVA pathway.
We have used MVA synthesis pathway which is common in plants. Since limonene and linalool have the same synthetic precursor GPP, we have divided the synthesis pathway into two parts:
1. (BBa_K3052001) IPTG inducible precursor circuit which contains eight enzymes of MVA pathway enable conversion from AcCoA to GPP: atoB, HMGS, HMGR, MK, PMK, PMD, idi, trGPPs.
2. (BBa_K3052004, BBa_K3052010) two Production Circuits: limonene synthase or linalool synthase regulated by a ptrc promoter in E. coli.
Reference [1]lonso-Gutierrez, J., Chan, R., Batth, T. S., Adams, P. D., Keasling, J. D., Petzold, C. J., & Lee, T. S. (2013). Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metabolic engineering, 19, 33-41.
Characterization
Ptrc and CS genes was obtained by PCR flanked two Type IIS sites BsaI and was used for Golden Gate Assembly to construct the expression vectors. The length of limonene synthase CS is 1632 bp. Here is the gel results confirming success amplification.
The expression of limonene synthase and linalool synthases are under the regulation of Ptrc. RT-qPCR and SDS-PAGE were conducted to test their expressions, as well as Gas Chromatography analysis was used to detect limonene yields. Click to see our protocol. We measured the transcription level of CS and LSs of four different strains, E.coli harboring only pGPP vector, E.coli with dual plasmids pGPP+pDHJ (Stain B in Fig 3), pGPP+pLGF (Stain C in Fig 3), pGPP+pCS (Stain A in Fig 3),and relative mRNA levels compared with the blank strain only harboring pGPP are shown in Fig 4.
As shown in Fig 4, compared to blank strain, the relative mRNA level of three strains were much higher, and clearly showed the efficient transcription and expression of CS gene in engineered E.coli. We also conducted SDS-PAGE of whole cell proteins to ensure its expression. As shown in Fig 6, compared to blank strain only with pGPP vector, CS protein was expressed but yields were not high, and because of the leaky expression, expression level with IPTG induction were just a little bit higher than the level without IPTG induction.
Both the results of above RT-qPCR and SDS-PAGE demonstrate the expression of the limonene synthase CS.
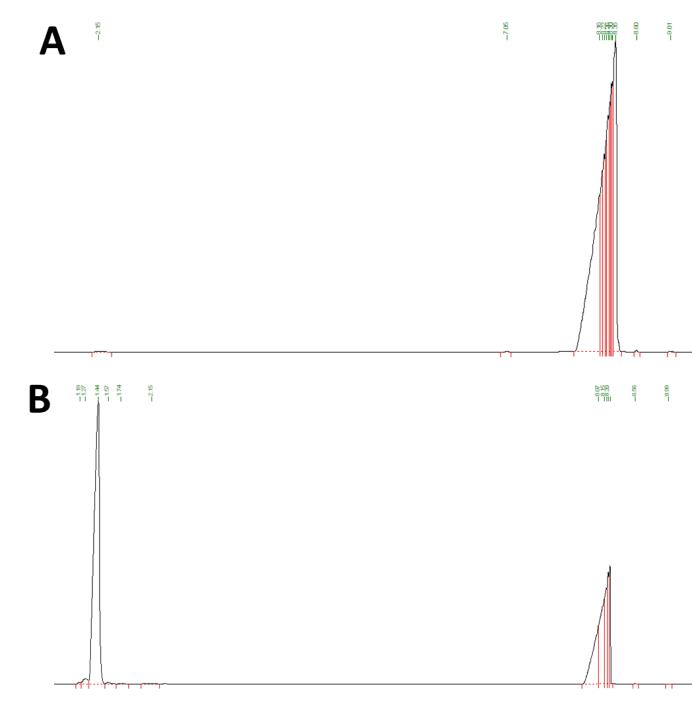
GC analysis will confirm whether the expressed protein is functional. Firstly we analyzed a positive limonene-containing control that yielded a strong peak as indicated by the black line with the retention time abound 1.44 minutes. The results show that there is no overlap of retention time between n-Hexadecane and limonene, and indicates the GC method is effective for testing. In comparison, we expressed the limonene generator with trc promoter in DH5α E. coli and lysed the cell culture, extracted with n- hexadecane, and the extraction was subjected to gas chromatography mass spectrometry.

The results show that the retention time of limonene is more clear at 1.37 minutes after optimization when the amount of limonene is small. It shares the same retention time with our experimental result of samples from E.coli that is transformed with pGPP and pCS. And the important GC-MS result showed the the MS profile is exactly the same with compound library and literature of limonene, further confidently confirming its production(See Fig below). This result indicates that GPP production can be achieved by precursor circuit, and most importantly, our limonene synthase can work properly to convert GPP to limonene even though the yield of limonene remains low, which needs to be further improved by more metabolic engineering of the strain.

The results indicate that the MS profile is exactly the same with compound library and literature of limonene,confirming its production. Based on the the standard curve, the yield of limonene in our project was around 1.49 mg/L.
| None |