Part:BBa_K2961003
gRNA / crRNA fixed-loop repeat for Cpf1 / Cas12a
Sequence (crRNA repeat) that all Lba Cas12a gRNAs need at the beginning. Also known as fixed loop
Characterization by iGEM21_GreatBay_SZ
- Group: iGEM21_GreatBay_SZ
- Author: TianYi Huang
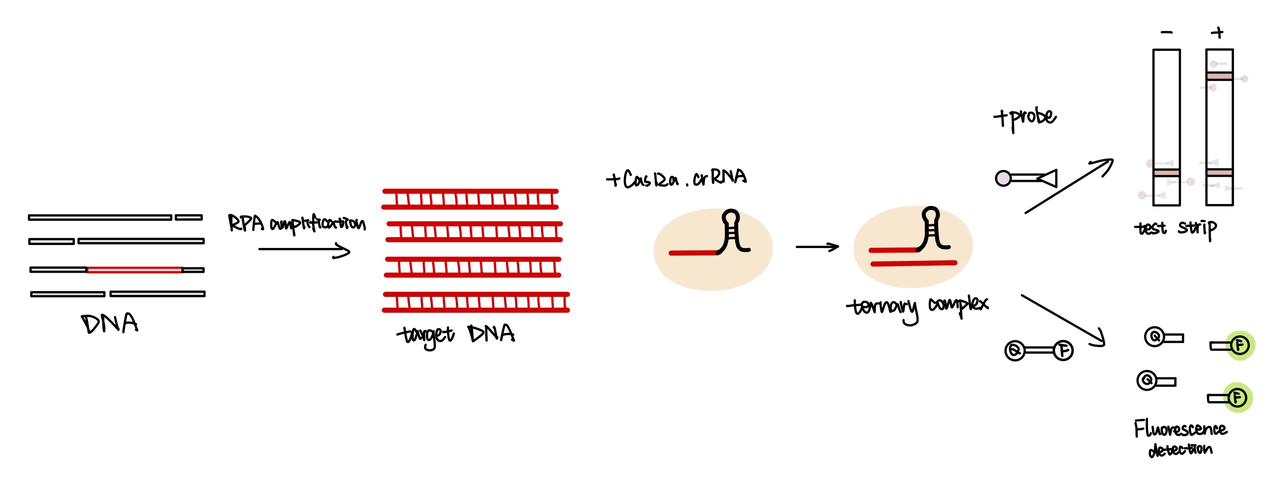
We attached the repeat sequence to guide RNA used to recognize their targets(Part:BBa_K3859013). The crRNA will bind with cas12a enzyme, then the target DNA will be recobgnised and unwound by cas12a. After target DNA binds with guide RNA, cas12a will be activated and cleveage the sequence around it. In our project, we primarily used Cas12a detection technology for the identification of spores. This decision was made with reference to the methodology used in the HOLMES system
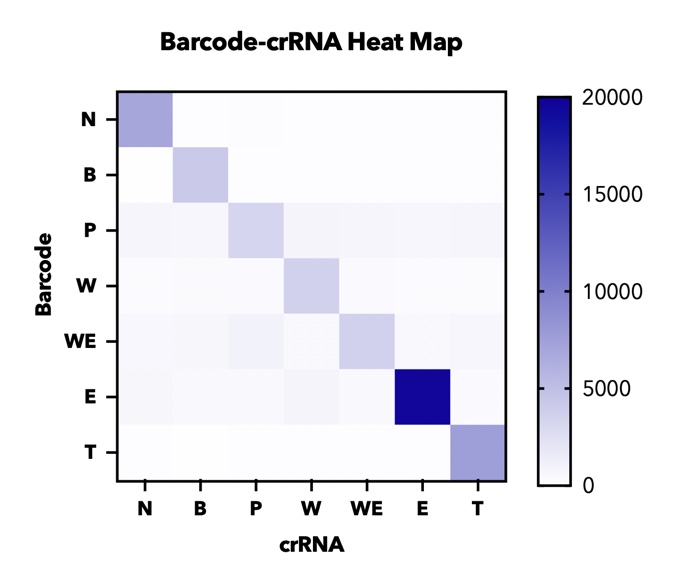
In addition, in order to test the specificity of our barcode design. We constructed 7 barcodes and their matching CRISPR RNAs (crRNAs) and assayed all permutations in vitro using cas12a florencence detection(fig.4).
Experienment and result from iGEM21_GreatBay_SZ
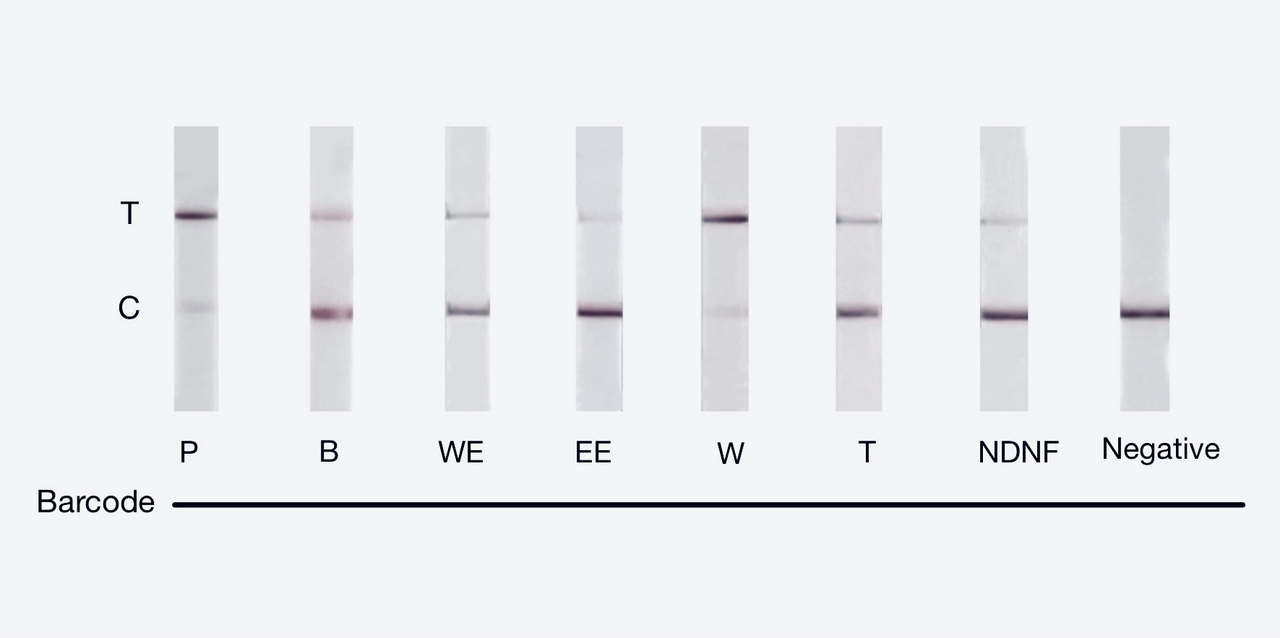
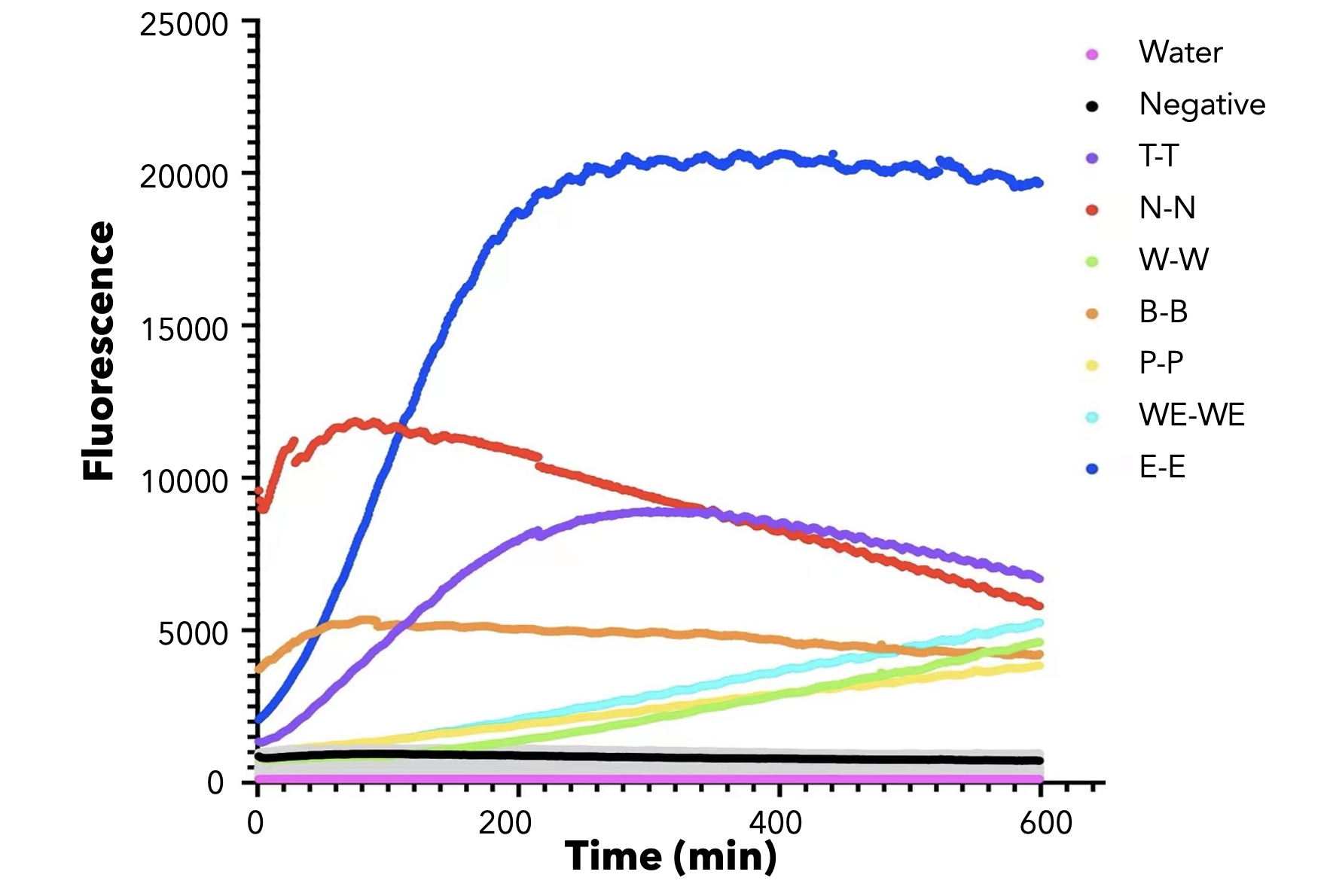
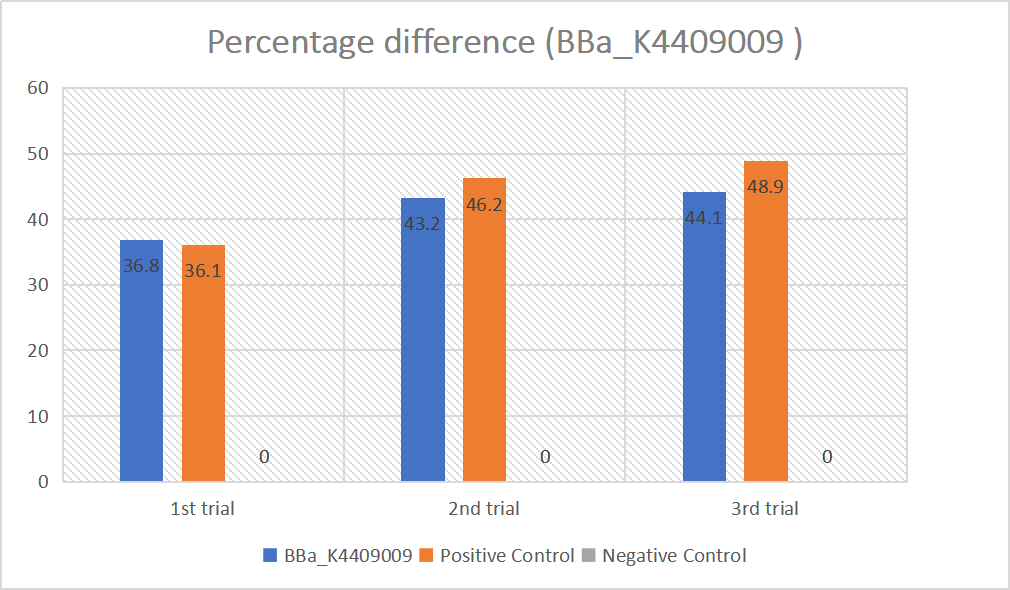
We used both the test strip and the florencence method in vitro. For the test strip, we successfully got the correct result for all the barcodes(fig.2). For the florencence method, we got the graphs of the florencence against time(fig.3), which were proofed right by our model.
【fig.2】Cas12a test strip result
【fig.3】Cas12a fluorescence detection result. On the left of the symbol "-" refers to barcode name; On the right of the symbol "-" refers to crRNA name. All results for barcode mismatch with crRNA are shown in grey. E.g. "T-T" means the mixture of T-barcode and T-crRNA.
In addition, in order to test the specificity of our barcode design. We constructed 7 barcodes and their matching CRISPR RNAs (crRNAs) and assayed all permutations in vitro using cas12a florencence detection(fig.3)[2].
【fig.4】Heatmap of endpoint fluorescence values from in cas12a fluorescence detection of all combinations of 7 barcodes and 7 crRNAs assessing specificity of each barcode-crRNA pair.
Characterization by iGEM22_Worldshaper-HZ
- Group: iGEM22_Worldshaper-HZ
- Author: Haoyu Xu
- Summary:Characterization of BBa_K2961003 working efficiently as a Cas12a handle region in three guide RNAs for a CRISPR-Cas12a system.
In this experiment, our team has tested and verified the efficiency of part BBa_K2961003 designed by iGEM19_CMUQ.[ https://parts.igem.org/Part:BBa_K2961003] We incorporated this sequence into three guide RNAs, including igRNA (AAG+GAPDH as guide+ hsa_circ_0001982 as trigger) (BBa_K4409009), gRNA (AAG+ BBa_K3859000) (BBa_K4409015), and gRNA (AAG+ GAPDH as guide)(BBa_K4409016), and used it as the binding site with Cas12 enzyme. By using a CRSPR-Cas12 system for these guide RNAs, our experiments showed that BBa_K2961003 was sensitive as a Cas12a handle region for these guide RNAs.
Experiment results
BBa_K2961003 in igRNA (AAG+GAPDH as guide+ hsa_circ_0001982 as trigger)
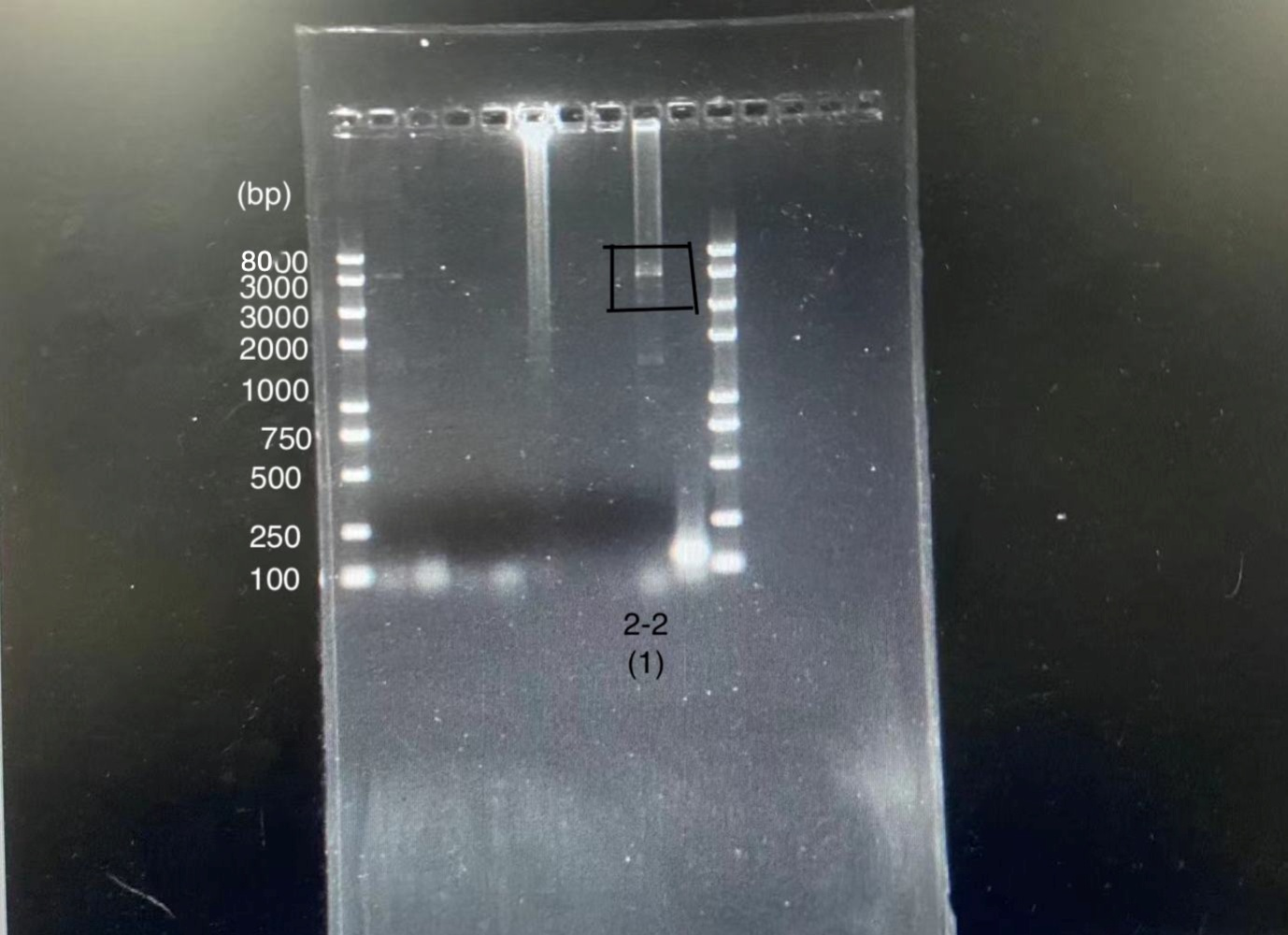
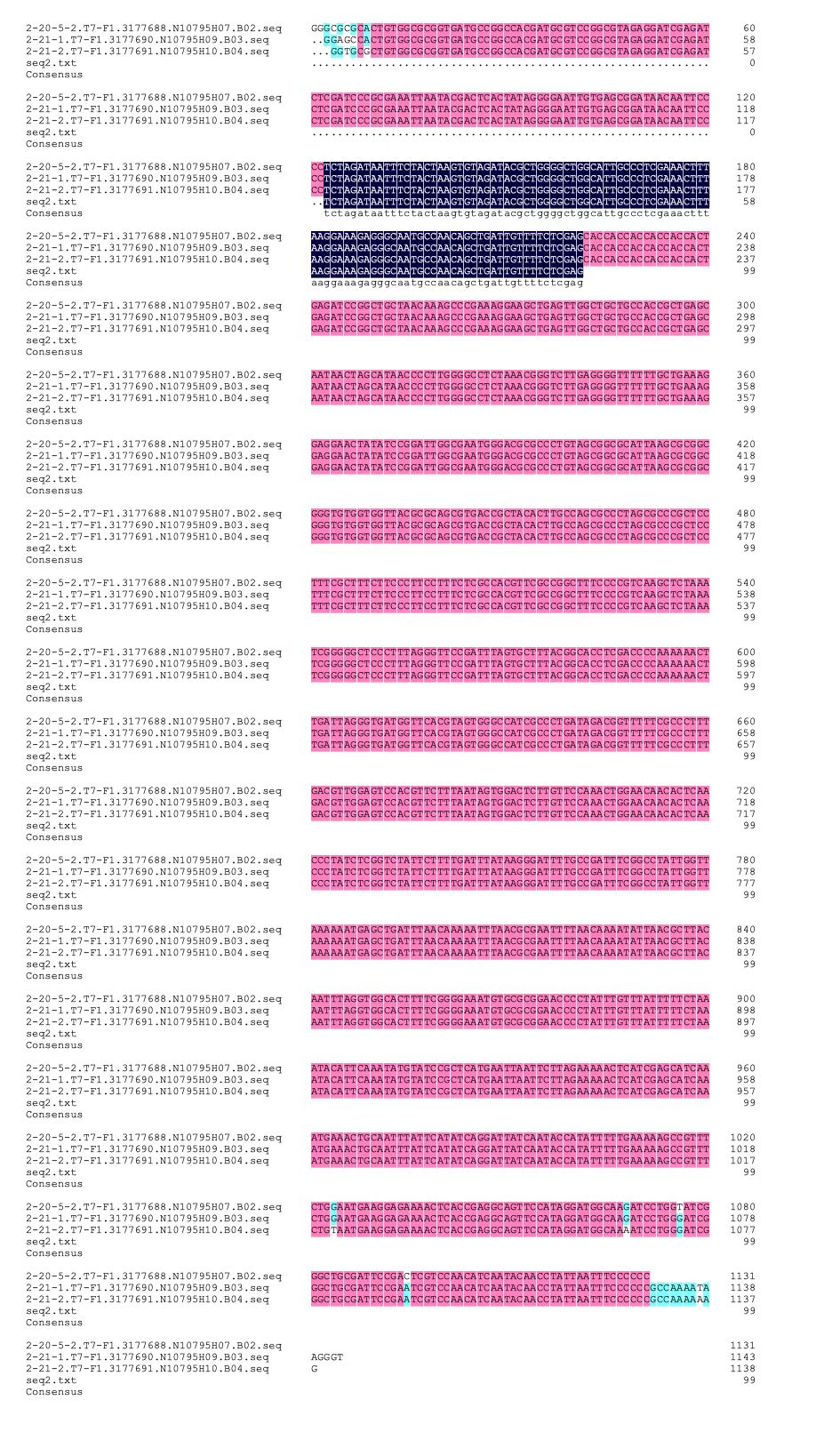
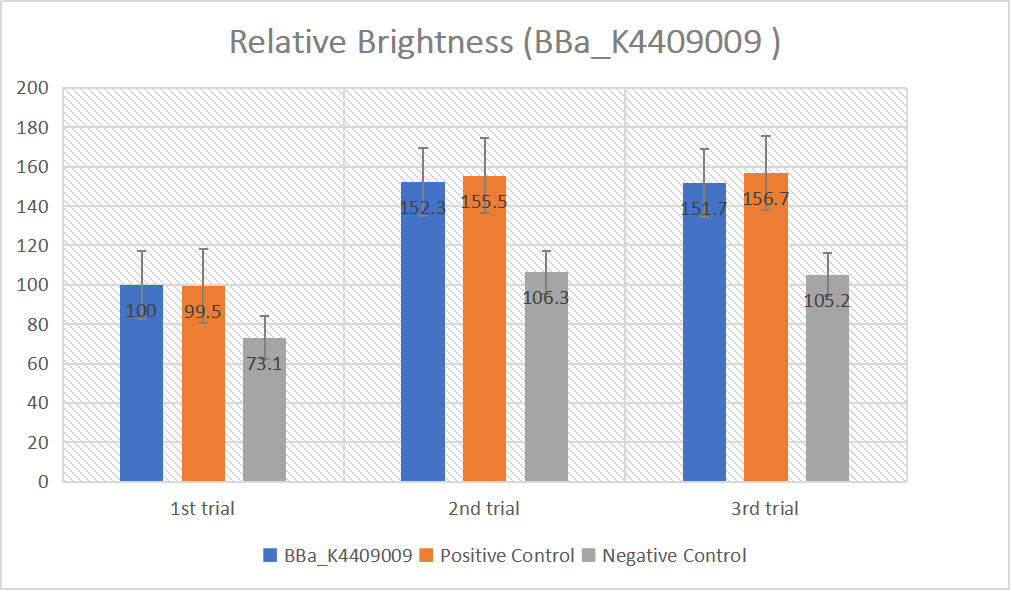
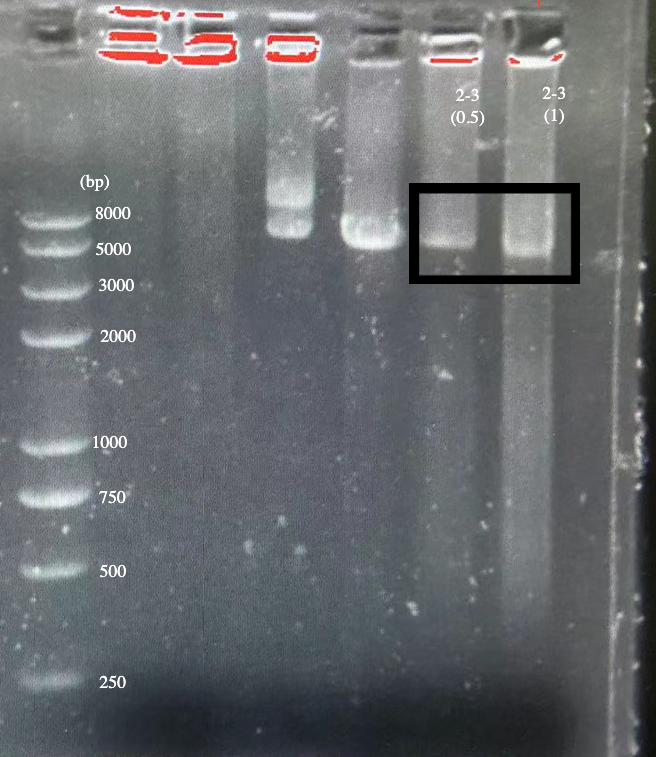
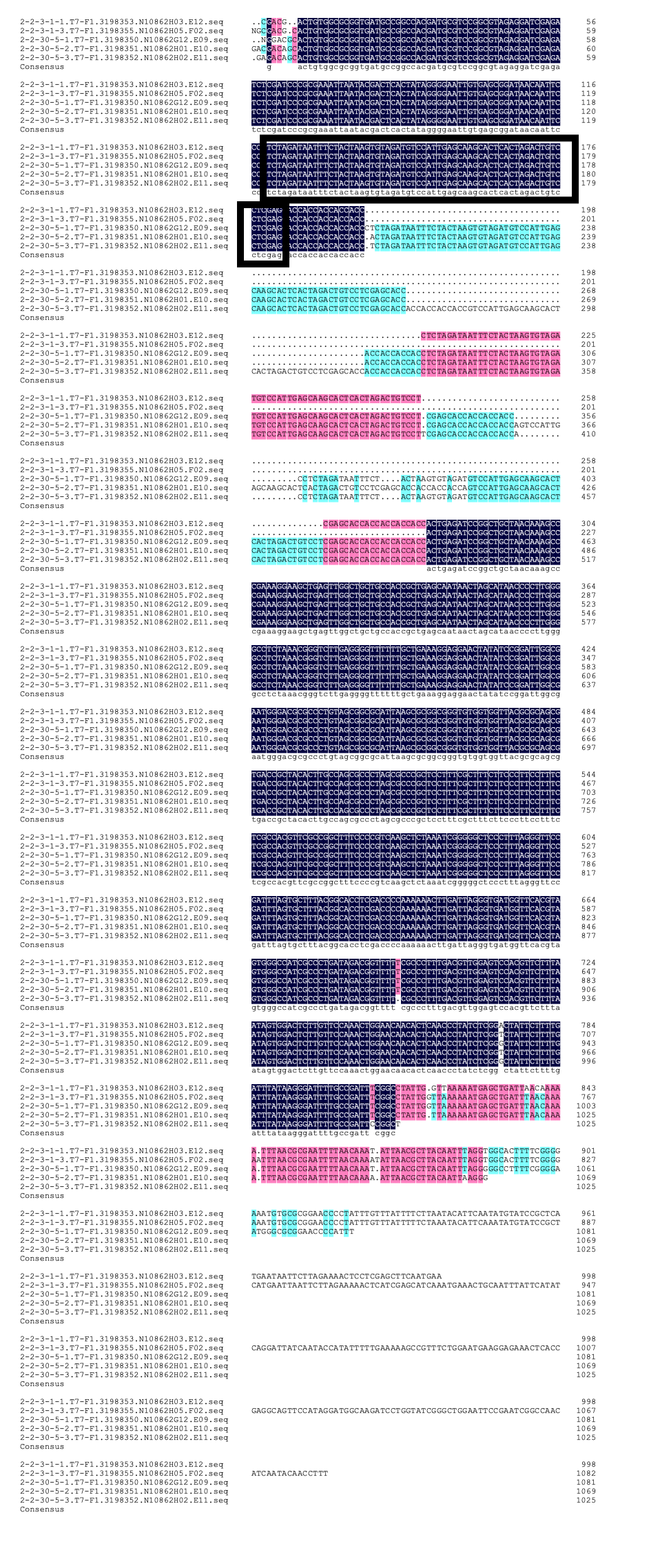
We designed igRNA (AAG+GAPDH as guide+ hsa_circ_0001982 as trigger) with BBa_K2961003 as the Cas12a handle to detect hsa_circ_0001982,and with GAPDH as its dsDNA substrate. For more information of igRNA (AAG+GAPDH as guide+ hsa_circ_0001982 as trigger), please refer to part BBa_K4409009. We constructed a pET-28a plasmid containing this igRNA (AAG+GAPDH as guide+ hsa_circ_0001982 as trigger). PCR and gene sequencing results showed the plasmid was constructed successfully (Figure 1-2). A CRISPR-Cas12 system using this igRNA was used to detect the circRNA hsa_circ_0001982, and it produced fluorescence significantly brighter than negative control groups. The fluorescence produced is shown in figure 3 and table 1-2. This showed that BBa_K2961003 was sensitive as a Cas12a handle region for this igRNA.
BBa_K2961003 in gRNA (AAG+ BBa_K3859000)
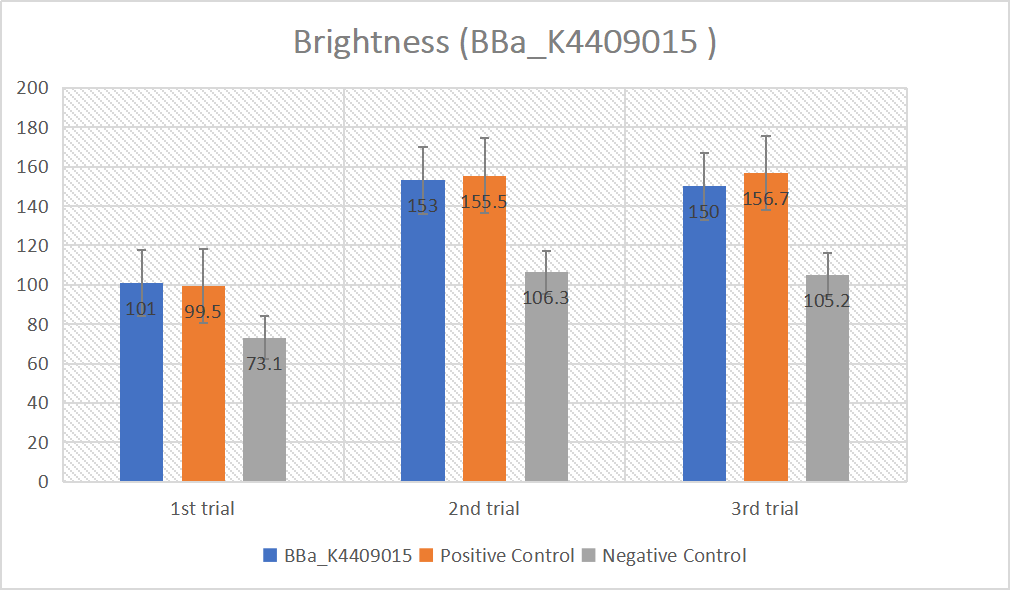
gRNA (AAG+ BBa_K3859000) mainly comprises a Cas12 handle (BBa_K2961003) and a guide sequence (BBa_K3859000) which binds with a dsDNA to activate the Cas12 enzyme. For more information of gRNA (AAG+ BBa_K3859000), please refer to part BBa_K4409015. We constructed a pET-28a plasmid containing the gRNA (AAG+ BBa_K3859000). PCR and gene sequencing results showed the plasmid was constructed successfully (Figure 4-5). A CRISPR-Cas12 system using this gRNA produced fluorescence significantly brighter than negative control groups. The fluorescence produced is shown in figure 6 and table 3-4. This showed that BBa_K2961003 was sensitive as a Cas12a handle region for this gRNA.
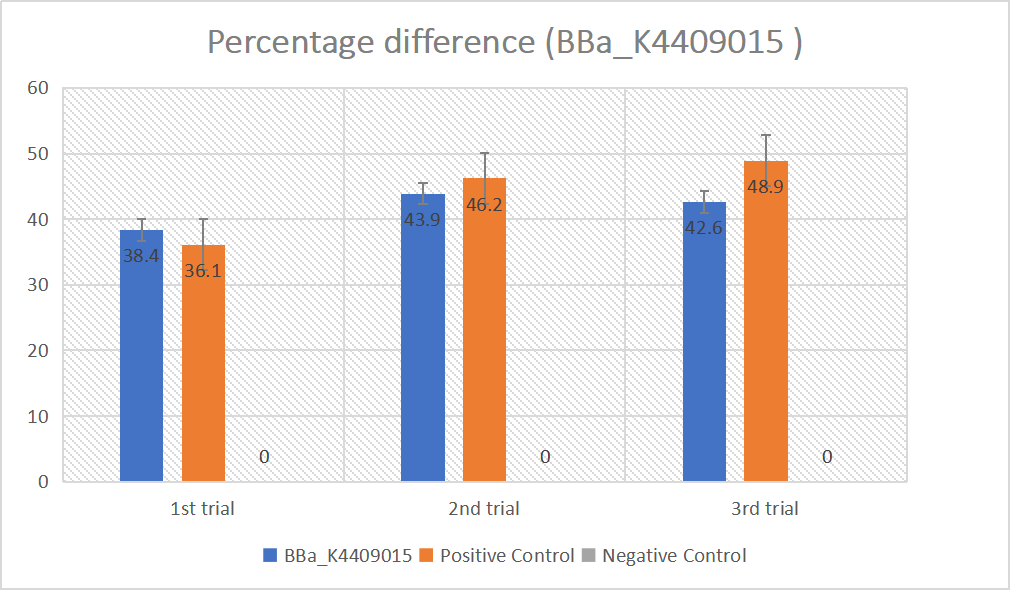
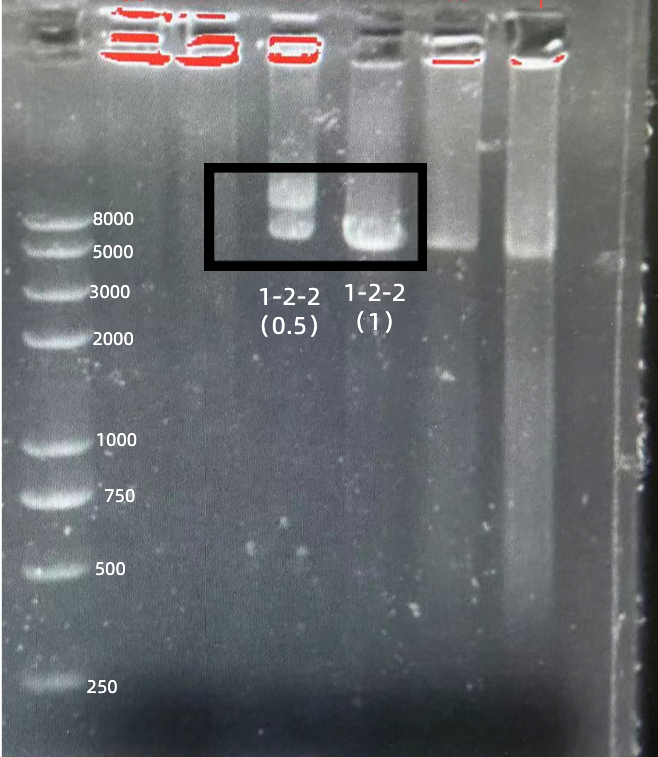
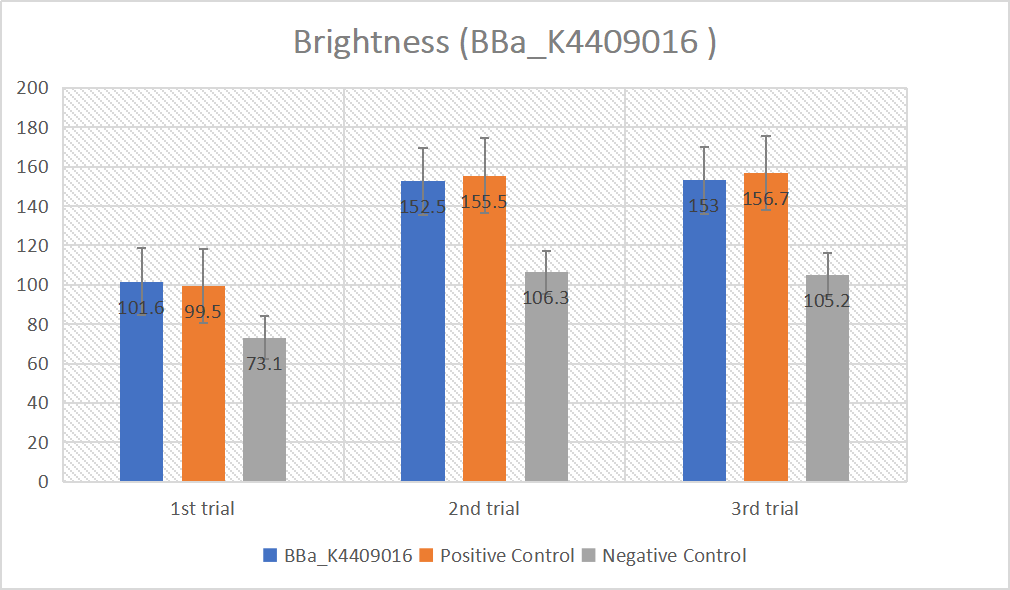
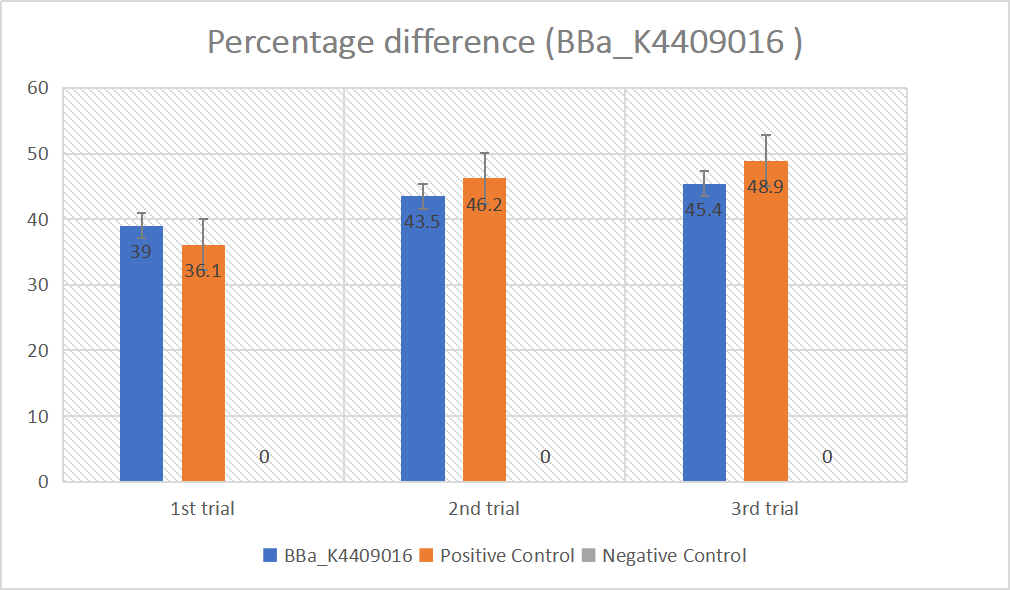
BBa_K2961003 in gRNA (AAG+ GAPDH as guide)
gRNA (AAG+ GAPDH as guide) mainly comprises a Cas12 handle (BBa_K2961003) and a guide sequence which binds to GAPDH dsDNA to activate the Cas12 enzyme. For more information of gRNA (AAG+ GAPDH as guide), please refer to part BBa_K4409016. We constructed a pET-28a plasmid containing the gRNA (AAG+ GAPDH as guide). PCR and gene sequencing results showed the plasmid was constructed successfully (Figure 7-8). A CRISPR-Cas12 system using this gRNA produced fluorescence significantly brighter than negative control groups. The fluorescence produced is shown in figure 9 and table 5-6. This showed that BBa_K2961003 was sensitive as a Cas12a handle region for this gRNA.

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |