Part:BBa_K1980001
TAT Copper Storage Protein 1 sfGFP
Description
Copper Storage protein 1 (Csp1) is a tetrameric copper storage protein found in the periplasm of Methylosinus trichosporium OB3b. We investigated whether this part could act as a copper chelator when expressed in E. coli. We modified the protein by adding a TAT signal peptide from the E. coli enzyme CueO in place of the native TAT sequence in an attempt to direct the protein to the periplasm and added a C terminal sfGFP fluorescent protein with a C terminal his tag. The Csp1 and sfGFP are separated by a short hydrophilic, flexible linker. (GlySerGlySerGlySer)
Sequence and Features:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 886
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 448
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Our project aimed to detect and chelate dietary copper as a treatment for Wilson's Disease, a copper accumulation disorder. We decided that the ideal copper chelation protein would have these properties:
- Should be able to bind multiple copper ions per peptide to increase the efficient use of cell resources.
- They should be from the prokaryotic domain because eukaryotic proteins can have expression issues in Escherichia coli.
- As E. coli naturally deals with copper toxicity by binding copper in the periplasm then exporting it, periplasmic proteins may reduce toxicity to the host.
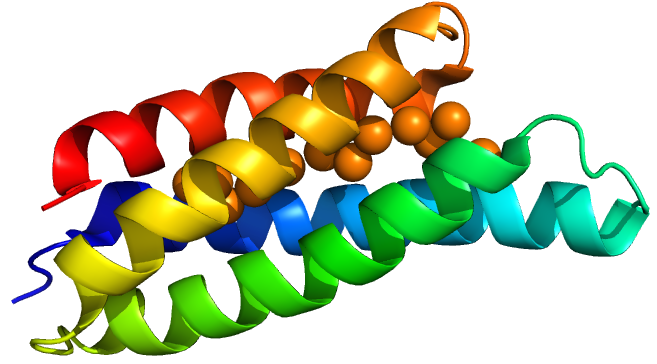
Copper storage protein 1 is a protein discovered in a methane-oxidizing alphaproteobacterium called Methylosinus trichosporium OB3b. (OB3b here stands for “oddball” strain 3b). This bacterium has a high demand for copper for use in its particular methane monoxygenase enzyme. Vita et al.(1) discovered Csp1 in 2015, characterised the protein’s copper affinity and obtained crystal structures with and without copper.
Csp1 is a tetramer of four-helix bundles. Each monomer can bind up to 13 Cu(I) ions meaning that the tetramer binds a maximum of 52 copper ions. Vita et al. crystallised Csp1 with and without copper bound. The copper is bound inside the pre-folded helical bundles by Cys residues in contrast to metallothioneins, which are unstructured until they fold around metal ion clusters. Vita et al. found an average copper affinity of approximately 1x1017M-1.
Csp1 has a signal peptide targeting it to the twin arginine translocation pathway (TAT). This means that it is likely a periplasmic protein. However they also found cytoplasmic homologues in many species challenging their and our assumption that only copper storage occurs in the periplasm due to copper toxicity.
We codon optimised Csp1sfGFP to E. coli and replaced the original TAT sequence with a TAT sequence from the E. coli protein CueO, which is also involved in copper regulation. To get Csp1sfGFP from the shipping vector to the pBAD expression system for testing the TAT sequence had to be modified by the addition of a serine residue after the initiator methionine. Serine was chosen over other amino acid possibilities because other TAT sequences seemed to have serine in this location.
Experience
We cloned Csp1sfGFP from Gblock into the shipping vector then transferred it into the pBAD, arabinose-inducible commercial expression vector. This transfer necessitated the changing of the TAT sequence by the addition of a serine residue after the initiator methionine.
Absorbance Assay
We were unable however to detect copper chelation activity of MymT when expressed from pBAD in MG1655 E. coli strain, using an absorbance assay.
The assay used was the reagent Bathocuproine disulphonic Acid (BCS). BCS is colourless in the absence of Cu+ but upon exposure to Cu+, BCS forms complexes with Cu+ and absorbs strongly in the 480nm range. In our assays, at concentrations of 50µg/mL, the 480nm absorbance varied linearly with the Cu+ concentration from the detection limit of around 1µM Cu+, to approximately 20µM Cu+. As not only MymT and Csp1 bind copper in the Cu+ form, but the assay also requires singly charged copper, the assays were optimised to include a suitable about of mild reducing agent to ensure reduction of the added CuSO4 (releases Cu2+) to Cu+. After trying L(+)-Ascorbate and DL-Dithiothreitol, and L-Glutathione as candidates, L-Glutathione was selected as it was both mild enough to not damage biological material and efficient at reducing Cu2+. At >2-3 times the concentration of Cu2+ in solution, L-Glutathione had maximum reductive activity against Cu2+.
Modelling by our team suggested that this was because insufficient protein could be expressed to chelate the amount needed to be detectable on the assay (1μM detection limit).
We purified this protein, from MG1655 cells expressing Csp1sfGFP from pBad, using Nickel affinity chromatography followed by dialysis. However were unable to detect copper chelation with the assay in our purified extracts when compared to a his-tagged GFP control. This is possibly because the flexible linked was susceptible to cleavage by proteases in the lysate meaning the protein we purified lacked the chelator portion. We attached the his tag to the C terminus of sfGFP rather than the N terminus of Csp1 in order to preserve the TAT sequence.
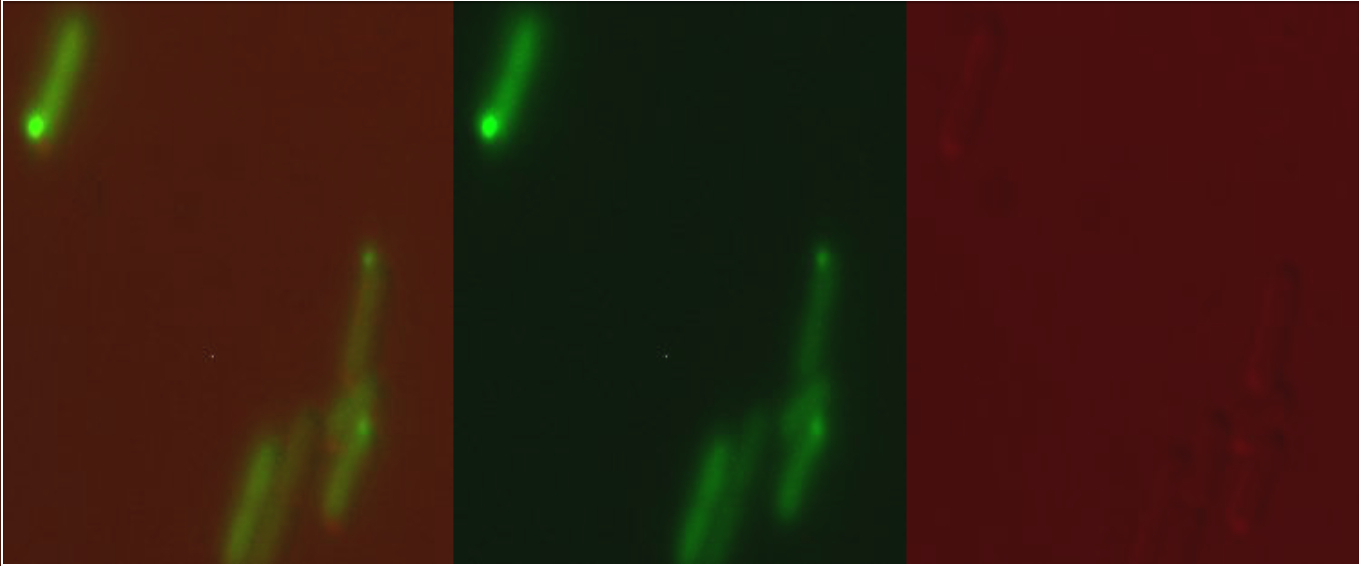
Microscopy
When expressed from the pBAD, arabinose-inducible commercial expression plasmid, or from our copper sensitive promoter (part BBa_K1980010) our version of Csp1sfGFP was observed to form inclusion bodies in the cytoplasm:
FLIM
We discovered a paper by Hötzer et al(2) that described how His-tagged GFP can be quenched by a copper ion binding to this His tag leading to a reduction in the fluorescence lifetime (the time the fluorophore spends in the excited state before returning to the ground state by emitting a photon.) They speculated that this could potentially be used as a in vivo copper assay.

As we had His-tagged our chelator-sfGFP constructs we were curious to see if this technique could be applied to our parts to measure copper chelation in vivo by our parts. We believe that two possibilities were likely:
- Copper chelation by the chelator reduces the free copper concentration inside the cell meaning that less binds to the His tag and the fluorescence lifetime will be greater than a His-tagged sfGFP control
- Copper chelation by the chelator would allow additional quenching if copper was bound within the quenching radius of the fluorophore leading to a reduction in fluorescence lifetime compare with a sfGFP control
Lacking access to a fluorescence lifetime microscope ourselves we contacted Cardiff iGEM who had a FLIM machine in their bioimaging unit.
We sent Cardiff iGEM our parts Csp1-sfGFP in pBAD and pCopA CueR sfGFP (as a control) in live MG1655 E. coli in agar tubes. Cardiff grew them overnight in 5ml of LB with 5μM copper with and without 2mM arabinose.
The imaging unit spread each strain on slides and measured the fluorescence lifetime of three areas on each slide.
(Acquisition parameters: using the x63 water immersion objective with excitation at 483nm (71% intensity, pulse rate 40MHz) and emission via a BP500-550 filter. Scan resolution at 512 x 512 pixels at pixel size of 0.26 microns/pixel, 1AU pinhole. Counts of >1000 per lifetime recording.)
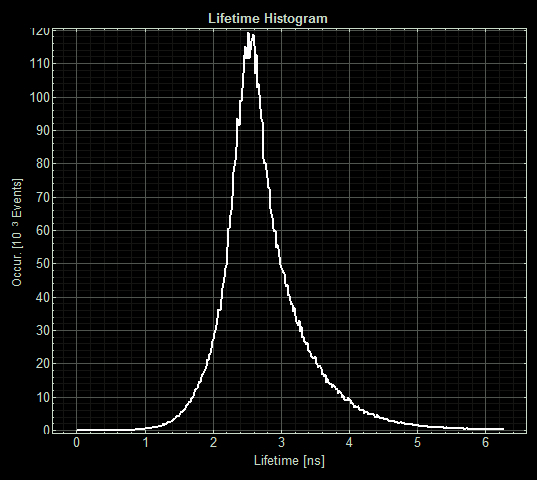
FLIM images from one section of each slide:

As expected the pCopA CueR sfGFP control was fluorescent, with and without arabinose, with the mean fluorescence lifetime a consistent 2.6ns.
When Csp1-sfGFP was induced the mean lifetime decreased to 2.5ns and the variance was increased. This might be indicate that copper chelation has occurred or may be reflective of the expression problems of Csp1.
References
(1) Nicolas Vita, Semeli Platsaki, Arnaud Basle, Stephen J. Allen, Neil G. Paterson, Andrew T. Crombie, J. Colin Murrell, Kevin J.Waldron & Christopher Dennison (2015) “A four-helix bundle stores copper for methane oxidation”, Nature 525 issue 7567 pg. 140-143 doi:10.1038/nature14854
(2) Hötzer B., Ivanov R., Altmeier S., Kappl R., Jung G., (2011) "Determination of copper(II) ion concentration by lifetime measurements of green fluorescent protein." Journal of Fluorescence, 21(6), pp. 2143-2153. doi: 10.1007/s10895-011-0916-1
Author: Sam Garforth and Andreas Hadjicharalambous
//cds/reporter/gfp
| None |