Part:BBa_K1893001
Las receiver with GFP reporter (LasR+pLas+GFP)
This composite part is based on the Las quorum-sensing system from Pseudomonas aeruginosa. It consists of a Las receiver device and a GFP reporter that is activated in the presence of a 3O-C12-AHL signal.
Usage and Biology
Quorum sensing is a naturally occurring mechanism that certain strains of bacteria use to regulate gene expression in response to their population density. These bacteria secrete autoinducer signalling molecules, such as N-acyl homoserine lactones (AHLs), that bind to transcription factors to alter gene expression.
In this case, the constitutively expressed LasR transcriptional regulator (BBa_C0179) is activated by the binding of 3O-C12-AHL, an AHL quorum signal. The activated LasR regulator binds to a LasR-inducible promoter, pLas, upstream of a GFP reporter. As a result, GFP is expressed when the receiver device is induced with 3O-C12-AHL. We designed this part to characterize the activation range of the Las receiver device. We also characterised the cross-talk of the Las receiver device with other quorum-sensing systems, in order to determine its orthogonality.
Characterisation
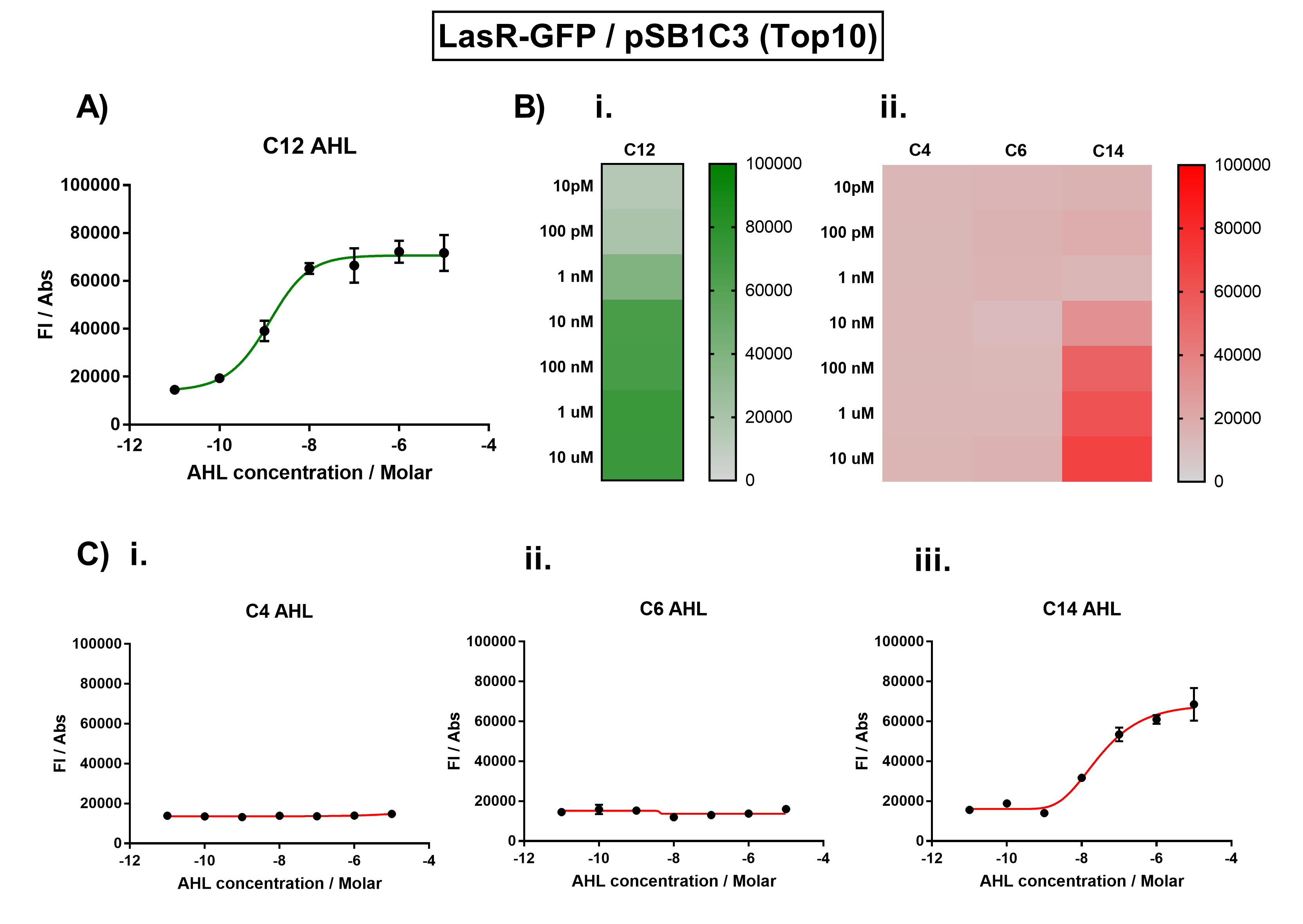
Figure 1. Characterisation of the Las response device (BBa_K1893001). (A) Transfer function curve of normalised fluorescence against cognate inducer C12-AHL (3O-C12 AHL) concentrations. (B) Heat map of normalised fluorescence of RhlR-GFP system over a range of AHL concentrations: (i) Binding of RhlR-GFP to its cognate AHL (3O-C12 AHL). (ii) Binding of RhlR-GFP to 3 non-cognate AHLs (C4 AHL, 3O-C6 AHL, 3OH-C14 AHL). (C) Transfer function curves of normalised fluorescence against non-cognate inducer AHL (3O-C12 AHL) concentrations to investigate inducer AHL crosstalk: (i) C4-AHL of the Rhl system (ii) C6-AHL (3O-C6 AHL) of the Lux system (iii) C14-AHL (3O-C14 AHL) of the Cin system. Experiments were performed in E. coli Top10 cell strain cultured at 37°C. Normalised fluorescence was calculated by dividing fluorescent signal by cell density (OD600). Fluorescence measurements were recorded at 180 minutes. Reported values represent the mean normalised fluorescence value from 3 technical repeats and error bars represent standard deviation of these.
The activation range of LasR by its cognate inducer (3O-C12-AHL) was characterised in TOP10 E.Coli cells. Cells transformed with the Las response device were cultured to the exponential phase and treated with appropriate concentrations of 3O-C12-AHL in 96-well microplates. These induced cells were grown in the microplates and their fluorescence and absorbance values (OD 600) were monitored over time using a microplate reader. The reported values for the normalised fluorescence represents the values recorded 180 minutes after AHL induction. The normalised fluorescence was calculated by dividing fluorescence values by absorbance values and correcting for LB autofluorescence.
In order to characterise the orthogonality of the Las system, we measured absorbance and fluorescence of the cells in the plate reader after treating them with varying concentrations of 3 different AHL signals (C4 AHL of the Rhl system, 3O-C6 AHL of the Lux system, and 3O-C14 AHL of the Cin system). This allowed us to determine whether these non-cognate AHLs were capable of activating the LasR response protein, and therefore the level of crosstalk between the quorum sensing systems.
The results from Figure 1A show that the concentration range of AHL (3O-C12 AHL) required for the activation of the Las response device was 100pM-10uM. Furthermore, it can be seen from Figure 1C that LasR does not appear to be activated by C4 AHL or 3O-C6 AHL, suggesting that the Las quorum system is orthogonal with Rhl and Lux. However, it is seen that LasR is activated significantly by 3O-C14 AHL, suggesting that the Las and Cin quorum systems lack orthogonality.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 383
Illegal AgeI site found at 580 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1719
| None |