Part:BBa_K1806005
T7+ ibPB RNA Thermometer + RFP
The part is designed to produce RFP in higher temperatures. The RNA Thermometer acts as a heat-shock RBS.
Heat-shock is a major process for the survival of all species.[1] In an environment where the temperature shows even the slightest inconsistency forces the organism to adapt to temperature shifts. This is where heat-shock proteins come into effect. Through sensing temperature shifts in the environment, organisms are able to survive and thrive in forever changing temperature conditions.
It has been understood that heat-shock mechanisms are required in almost all organisms and is present in the most primitive bacteria species to the most complex organisms.[2] This very rudimentary mechanism therefore necessitates to be handled by the most common structures found in all that is living, RNA. The heat sensitive mechanisms in living organisms are conducted by
RNA thermometers.
The Properties of RNA Thermometers RNA Thermometers are a group of RNA structures that have varying properties and operative functions. Although RNA Thermometers are basic and found in almost all organisms, characteristic differences exist in between RNA Thermometers. Between species, conformational, structural, functional and mechanical differences are present. This diversity allows the presence of many RNA Thermometer structures for application in different circumstances.
There are common features of RNA Thermometers. The main feature of all RNA thermometers is that they function through conformational shifts in structure. These shifts cause conformational changes to expose the Shine-Dalgarno sequence, which acts as a binding site to allow translation.[3] For translation to occur, the ribosome has to the aforementioned SD sequence. The structural differences are caused by the transcription regions, but the SD sequence is common. Aside from that, temperature is the factor responsible for changes in all RNA thermometers, but there are cold-shock RNA Thermometers as well as heat-shock.[4]
ibPB RNA Thermometer
The Rna thermometer that will be utilized in our project is the ibpb RNA thermometer. Taken from the strains of the x species of the x group of organisms, the ibpb thermometer is a standard cis-acting heat-shock regulated rna thermometer. The heat shock process is stimulated by the medium of the attached protein. This heat shock response is effectively stimulated at 37 °C and beyond, however the RNA thermometer starts to function at 32 °C.[5] 32 °C presents enough energy for some of the strands in the medium to start translation, while mostly, translation occurs at low levels. The unbinding of the dna sequence reaches its maximum at 37 °C, but the rate of the reaction and translation increases as the temperature further increases, as the number of ribosomes that can collusively attach to the SD sequence increase with temperature. This means that the activity of the strain increases with temperature, with the temperature of denaturation being the limiting factor.
Cloning
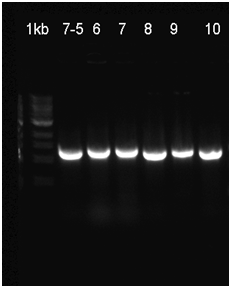
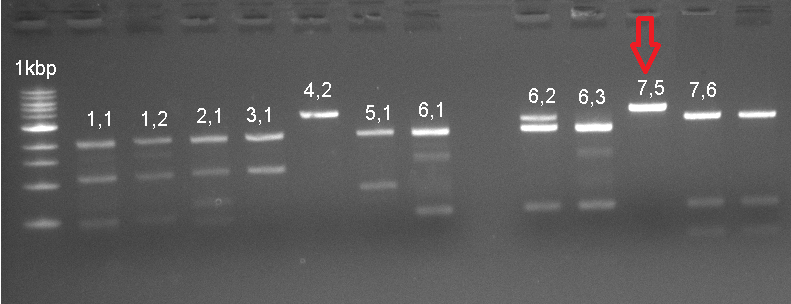
The already linear part was ligated to the pSB1C3 vector, and then transformated to be verified with Colony PCR. The RFP in the iGEM Registry contained Hind3, unlike the RFP in this part. For this reason, the part was cut with the EcoR1 and Hind3 restriction enzymes. The gel runs of the part were examined. The part was tagged as G-Block 7 and sample 7-6 yielded the accurate base pair length.
Functional Assay
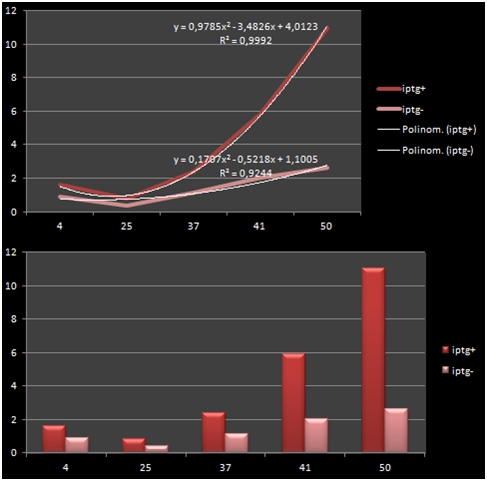
The bacteria that were transformated with G-Block 7 were cultured in 5 ml and incubated for 13 hours. After this incubation, the cultures were incubated at respective temperatures for 4 hours and were given 50uM of iPTG twice with 4 hours periouds. There were a total 5 different temperatures that the cultures were incubated in: 4, 25, 37, 42 and 50 C. The proteins in the cultures were then isolated and the protein concentrations were measured. Data on RFP concentration were acquired at 584 nm of emission and 607 nm of excitation. The acquired values were divided to the total amount of protein to acquire the following ratio.
| Medium Temperature | iPTG Presence +/- | Total Amount of Protein | RFP Fluorometric Measurement | RFP/Total |
|---|---|---|---|---|
| 4 | + | 14.09 | 22.32 | 1.584 |
| 4 | - | 11.349 | 10.36 | 0.912 |
| 25 | + | 17.87 | 14.35 | 0.802 |
| 25 | - | 11.054 | 4,313 | 0,390 |
| 37 | + | 16.808 | 40.2 | 2.391 |
| 37 | - | 15.216 | 17.36 | 1.140 |
| 41 | + | 7.637 | 44.83 | 5.870 |
| 41 | - | 5.557 | 11.13 | 2.002 |
| 50 | + | 5.463 | 60.06 | 10.993 |
| 50 | - | 7.997 | 20.95 | 2.619 |
The graph shows the variance in density concentrations of RFP, caused as a result of the functioning of the iPTG Inducible Promoters in different temperatures. The functioning of the promoters increased cumulatively with increased temperatures.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 2
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 720
Illegal AgeI site found at 832 - 1000COMPATIBLE WITH RFC[1000]
References
1 Guisbert, E., Yura, T., Rhodius, V.A., and Gross, C.A. “Convergence of molecular, modeling and systems approaches for an understanding of the Escherichia coli heat shock response.” Microbiol. Mol. Biol. Rev. 72 (2008): 545-554. doi:10.1128/MMBR.00007-08 2 Tripathy, Sindhu Nandini. "Molecular Biology of Endosalpinx." In The Fallopian Tubes, 31. New Delhi: Jaypee Brothers Medical Brothers, 2013. 3 Narberhaus F, Waldminghaus T, Chowdhury S. "RNA thermometers". FEMS Microbiol. Rev. 30 no.1 (2006):3–16. doi:10.1111/j.1574-6976.2005.004.x. PMID 16438677. 4 Jens Kortmann, Franz Narberhaus. “Bacterial RNA thermometers: molecular zippers and switches” Nature Reviews Microbiology 10 (2012):255-265 5 Shearstone, Jeffrey R., and Francois Baneyx. "Biochemical Characterization of the Small Heat Shock Protein IbpB from Escherichia Coli." THE JOURNAL OF BIOLOGICAL CHEMISTRY 274, no. 15 (1999): 9937-945.
| n/a | T7+ ibPB RNA Thermometer + RFP |