Part:BBa_K1639006
PotB59-pomA
PotB59, chimeric protein that is formed by the N-terminal region of PomB and the C-terminal of E. Coli MotB
It was shown that a chimeric protein, PotB, renamed as PotB59, that has the N-terminal region of PomB (1–50) fused to the C-terminal periplasmic region of E. coli MotB (59–308), is functional with PomA in E. coli as well as in strains of V. alginolyticus that are defective in MotX or MotY (Asai et al. 2003) Instead of the hydrogen-dependent MotA, MotB stator protein partially adapted from the V. alginolyticus, sodium-dependent Stator proteins were insalled. After this adaptation, the flagellar velocity increased by twice fold.
Usage and Biology
Mucus layer of stomach is very thick and the flagella of natural E. Coli does not have sufficient torque or speed to allow to enter.. In our project, we aim to give E. Coli the ability to enter the stomach’s mucus layer by increasing both of these characteristics. In order to achieve this goal, we plan to use PomA/PotB59 system.
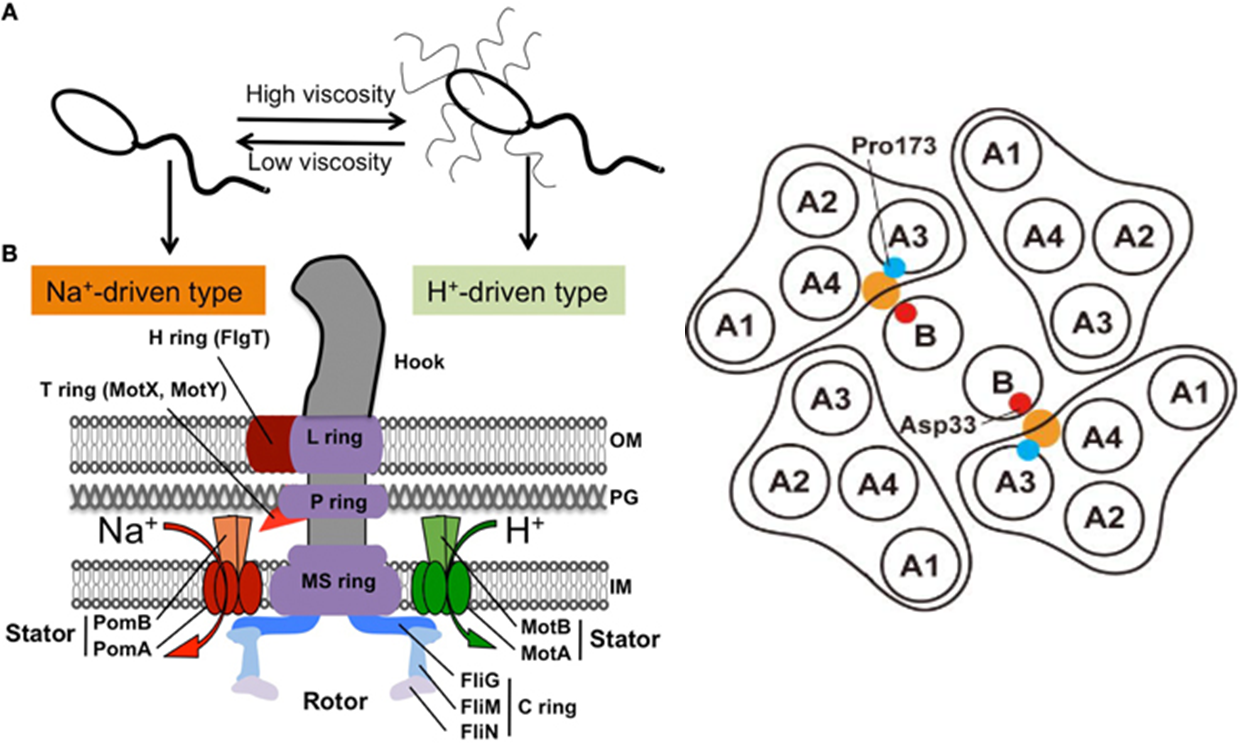
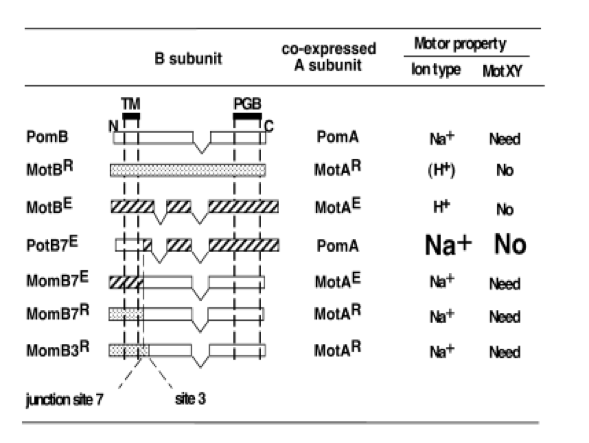
The driving force of the bacterial flagellar rotation is an ion concentration gradient between the two sides of the membrane. The energy needed to rotate the flagella is generated by the majority of bacteria as a H or Na ion concentration gradient. In proton(H)-driven motors, the rotation of the flagella is carried out by the MotA and MotB membrane proteins. This system is used by E. Coli. Other bacterial strains rely on Na+ to power their flagellar motor. These include Vibrio spp. These bacteria, in place of MotA and MotB proteins, use homologous membrane proteins PomA and PomB. These proteins function as Na+ channels and ensure that the necessary energy for flagellar rotation is generated as Na+ passes along the channel.(Figure 1) Flagella that work by Na+ have greater speed and rotational power that those that depend on H+
In work by M. Homma et al, a host of chimeric proteins were generated by combining different parts of the stator proteins (MotB and PomB) from the two systems mentioned above. According to their data, the most effective protein was (PotB59)(PomB7E) which was generated by combining the N-terminal tail of PomB with the periplasmic C-terminal tail of MotB and most effective rotor system was combination of PomA/PotB59.(Figure 2)
Results
Cloning and Expression:
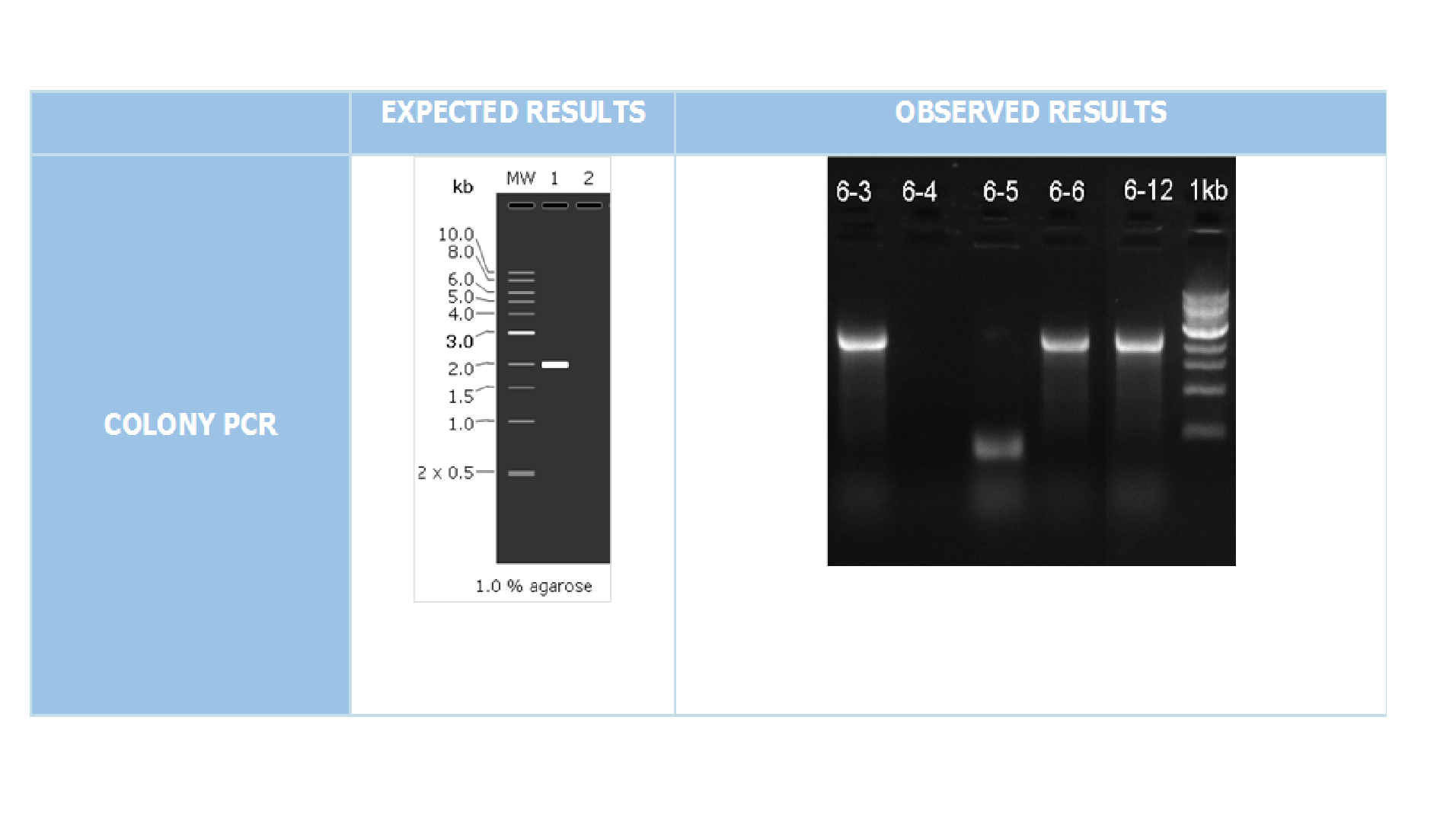
We cloned our gene into expression vector pET45-b with BamHI and XhoI enzymes. For confirmation colony PCR was performed with T7 Promoter Forward and T7 Terminator Reverse primers(Figure 3)
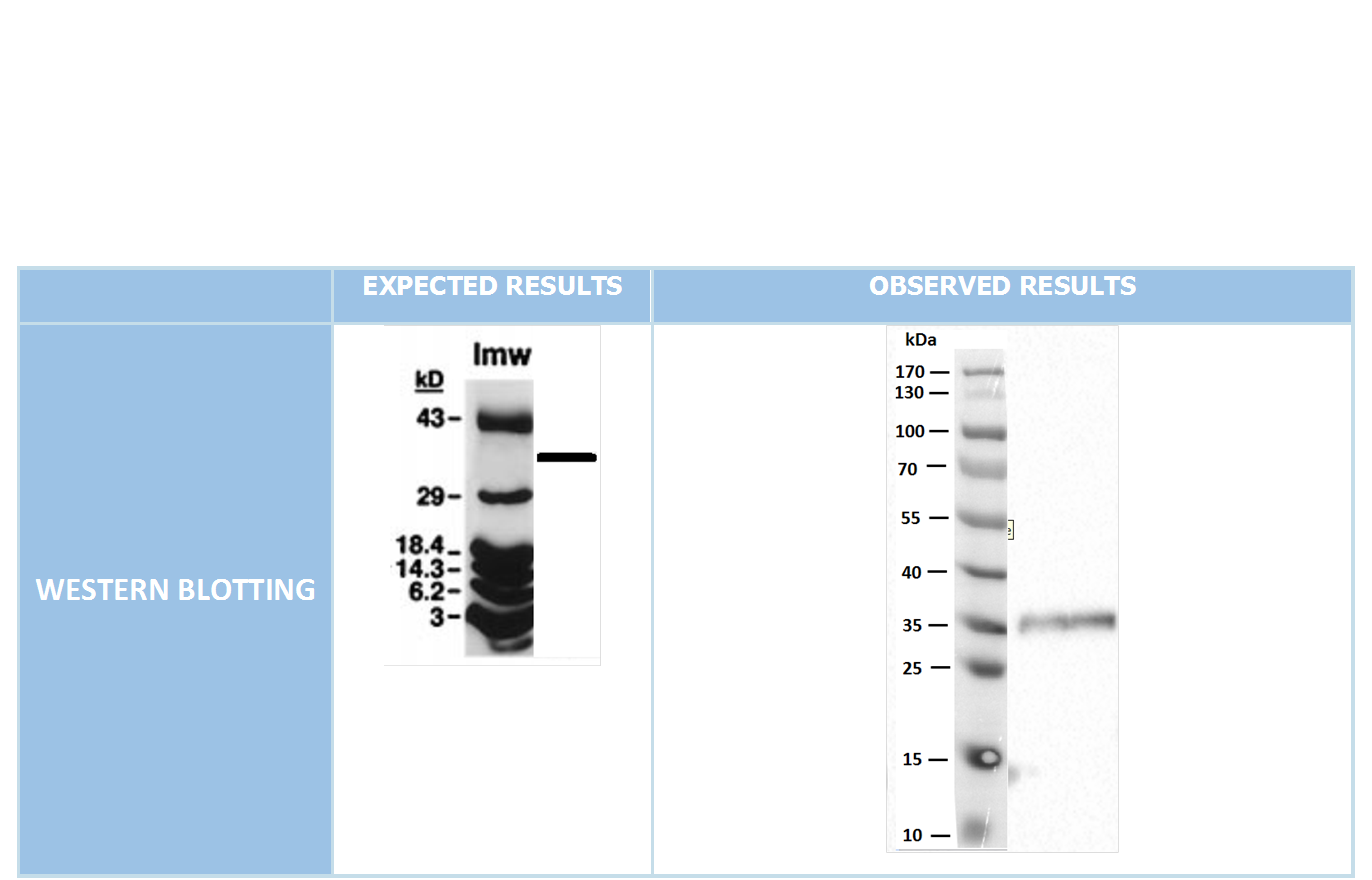
After cloning the genes into pET45-b successfully, we did Western Blot experiment through N-terminal located His Tag in proteins, so we managed to show the production of required proteins.(As we didn’t have any S-tag antibodies for Western blotting, the bands shown for PotB59/PomA indicate only the presence of PotB59 protein.(Figure 4)
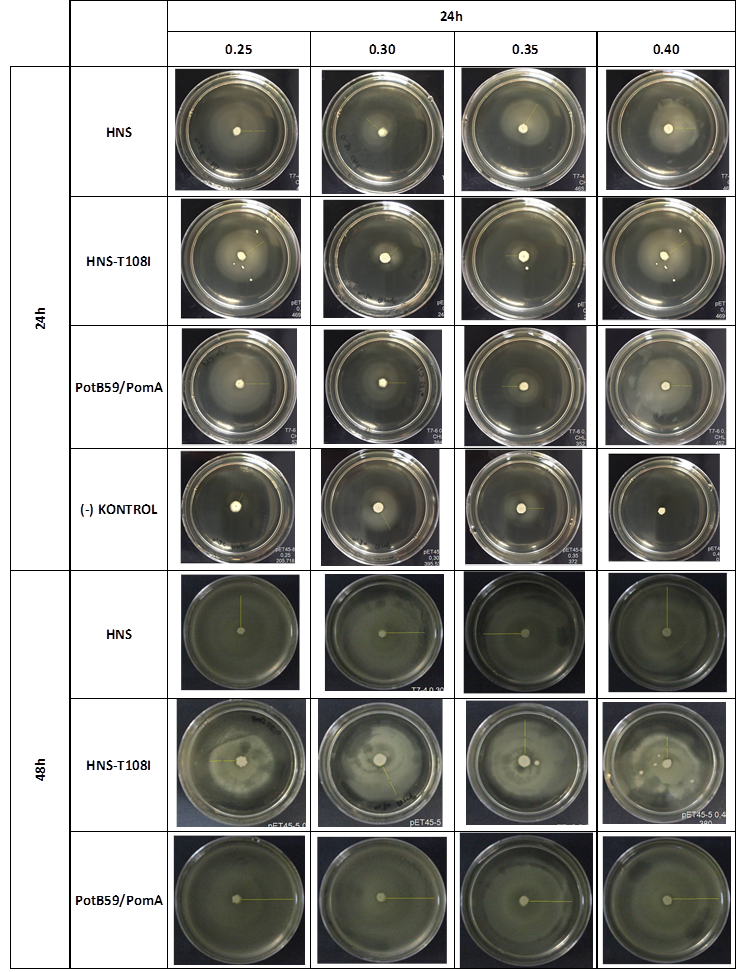
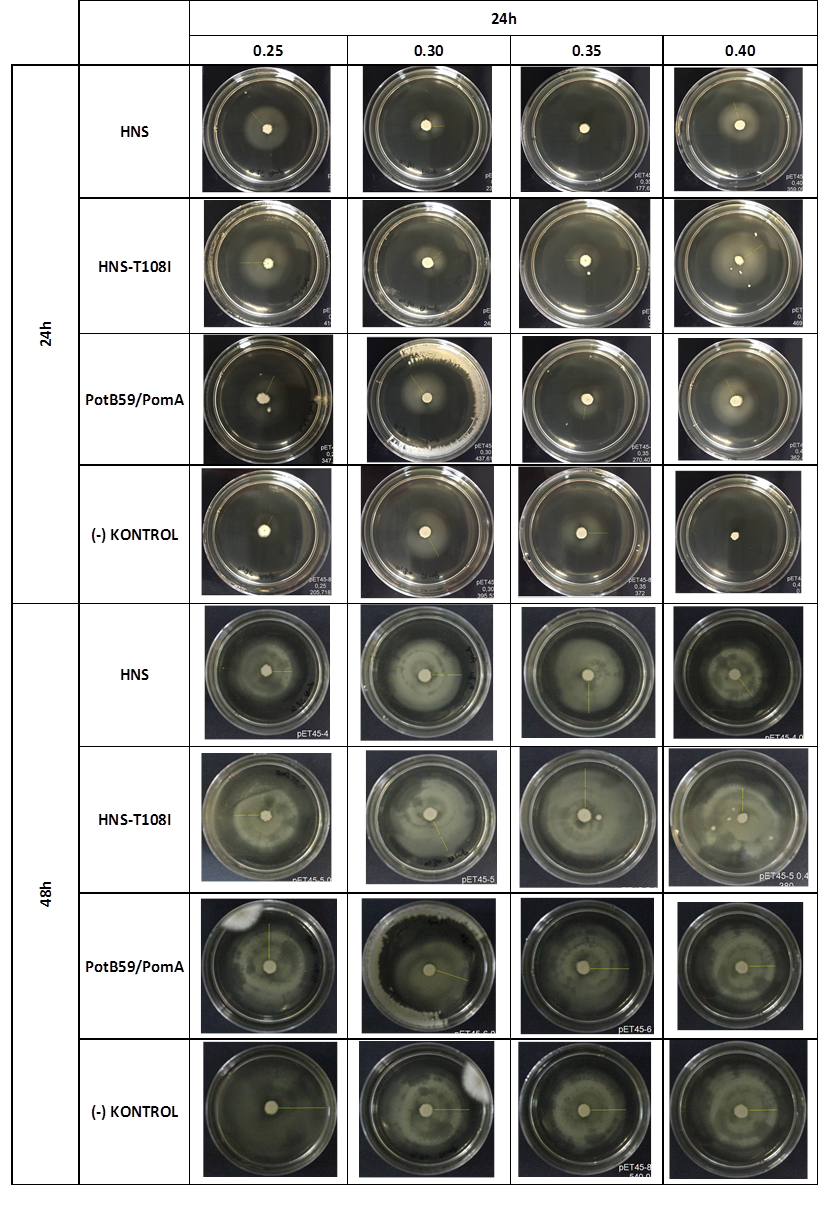
Soft Agar Plug Assay: We inoculated bacteria in different concentrations into the soft agars and incubated them at 37°C. After this, we measured their colony diameters at 24. and 48. hours. The results are shown below
The measurements of 0.25 and 0.4 concentrations in first 24. hour period, gives us significant positive results . If we compare the results,colony diameters are ordered like this; (-) kontrol < Wild type HNS < PotB59/PomA. The results of 0.30 and 0.35 agar concentrations doesn’t give significant information. Besides, in 48. hour of incubation the diameter of colonies reached to plate’s diameter, so measurement results didn’t show any difference than 24. hour’s.
We repeated functional assays after we cloned the genes into PSB1C3-T7. The results are shown below.
The measurements which made at first 24. hour period, gives us significant positive results . If we compare the results,colony diameters are ordered like this; (-) control < Wild type HNS < HNS-T108I< PotB59/PomA
//chassis/prokaryote/ecoli
//collections/probiotics/production
| None |