Part:BBa_K1342005
zinTp (Cd2+ sensing promoter) (Fw:Lac promoter(-) ver.)
Lac promoter (BBa_R0010) in the upper of the zinTp was removed from BBa_K896008.
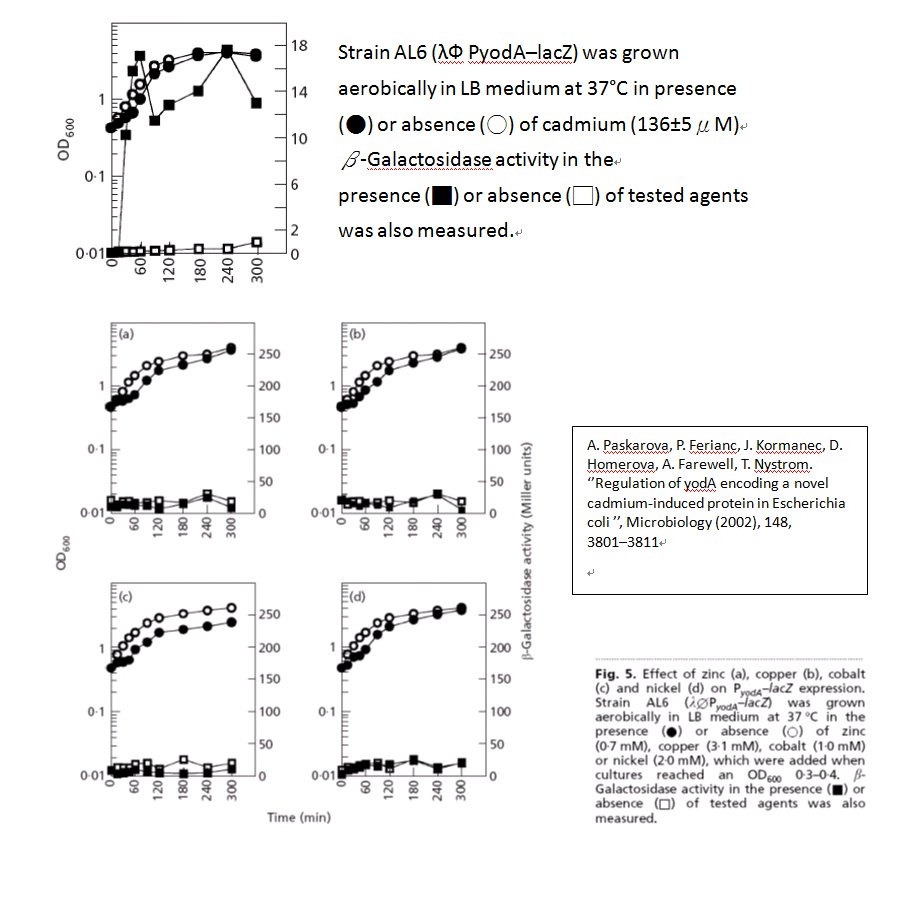
ThisZinTp (pYodA) is a promoter which expresses the downstream gene in the presence of cadmium ion. The activity of this promoter is specific affect by cadmium ion and won’t be induced by other ions like zinc, copper, cobalt, and nickel.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Contribution of FDR-HB_Peru 2022
In the presence of cadmium, The ZinT gene codes for the protein ZinTp as a stress response and metal assimilation mechanism. This means that it binds to cadmium and chelates it. However, according to the paper “Zinc dependence of Zint (YODA) mutants and binding of zinc, cadmium, and mercury by ZinT”, it can also effectively bind to mercury and zinc.
To analyze the reaction to different environmental conditions and the metal binding capacity of the ZinTp, they performed experiments on E. coli MG1655 and the E. coli MG1655 ZinT disruption mutant. One of the most important experiments was testing the binding capacity of the protein with three heavy metals. This consisted of using five different concentrations, 0.1, 0.2. 0.5, 1, and 2 mM, of either cadmium, mercury, or zinc. Then, the protein and metal mixture was incubated in a dialysis buffer for 2 hours before going through an electrospray ionization-mass spectrometry.
As we can appreciate in the figure, cadmium binding was observed down to 0.1 mM, which is the lowest concentration, while the binding of zinc and mercury was observed at a minimum of 0.5mM. Through these results, it can be understood that the protein has a higher affinity for cadmium, but it can work for the chelation of other metals.
Since ZinTp can be found in the periplasmic space and the cytoplasm of E. coli cells, its overexpression could potentially enhance the cadmium uptake of the bacteria and open up a new possibility for bioremediation of soil through hyperaccumulation.
Bibliography
Hobman, J. (2015, July 7). Zinc dependence of Zint (YODA) mutants and binding of zinc, cadmium, and Mercury by Zint. Biochemical and Biophysical Research Communications. Retrieved October 10, 2022, from https://www.academia.edu/13736354/Zinc_dependence_of_zinT_yodA_mutants_and_binding_of_zinc_cadmium_and_mercury_by_ZinT
Ferianc, P., Farewell, A., & Nyström, T. (1998, April 1). The Cadmium-Stress Stimulon of Escherichia Coli K-12. Microbiology. Retrieved October 10, 2022, from https://www.microbiologyresearch.org/content/journal/micro/10.1099/00221287-144-4-1045?crawler=true EcoCyc. (2022, June 21). Metal-binding Protein ZinT. Escherichia coli K-12 substr. MG1655 Zint. Retrieved October 11, 2022, from https://ecocyc.org/gene?orgid=ECOLI&id=G7061
| n/a | zinP (Cd2+ sensing promoter) (Fw:Lac promoter(-) ver.) |

