Part:BBa_K117002
LsrA promoter (indirectly activated by AI-2)
AI-2 quorum sensing system
The integral part of the AI-2 quorum-sensing (QS) system consists of a transporter complex, LsrABCD; its repressor, LsrR; and a cognate signal kinase, LsrK. Once uptaken into the cell, AI-2 will be phosphorylated under the effect of LsrK. Without the presence of AI-2, LsrA promoter is inhibited by the repressor, LsrR. Phosphorylated AI-2 will prevent LsrR from inhibiting this promoter, hence activating it so that the downstream sequence can be expressed. To prevent the cell from self-inducing this promoter, we must suppress its AI-2 producing capability. It can be done by knocking out LuxS, one of the vital gene required for producing AI-2. By doing that, LsrA promoter can only be activated under the presence of AI-2 from other sources than the host cell which is LuxS mutant (say, by contact with normal cells).
More information on AI-2 quorum-sensing (QS) system please refer to: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1952038
Modelling The Lsr Promoter - Contribution by Manchester iGEM 2021
As the Lsr promoter is a useful part to many iGEM teams, we decided to add some information on a series of differential equations which can be used to model the production rate of the Lsr promoter. With this information, teams can begin to build models around AI-2 inducible systems (source here: https://doi.org/10.1371/journal.pcbi.1002172). To use these, teams must estimate a value of extracellular AI-2 concentration. For our own project we also assumed that under control of the Lsr promoter the concentration of our protein produced would be the same as the concentration of the Lsr operon proteins. We also created equations to model bacterial population and AI-2 production which can be found here: https://2021.igem.org/Team:Manchester/Model. It should be mentioned that the concentration given is an intracellular concentration so any secreted proteins must undergo further modelling or assumptions.
Variables
| Symbol | Description | Initial Value |
|---|---|---|
| t | Time | 0 minutes |
| [OP] | Lsr-operon concentration | 0 M |
| [REG] | Lsr Regulator Cocnentration | 0 M |
| [R] | LsrR Concentration | 0 M |
| [Ap] | Intracellular Phosphorylated AI-2 concentration | 0 M |
| [Aout] | Extracellular AI-2 Concentration | Estimate/model this uM |
Parameters
| Parameter | Description | Value |
|---|---|---|
| kop | Lsr-synthesis rate | 7 uM-1 min-1 [2] |
| kr | LsrR synthesis rate | 2 min-1 [2] |
| k1 | Repression Coefficient (Lsr-operon) | 0.2 uM [2] |
| k2 | Repression coefficient (LsrR) | 0.1 uM [2] |
| k3 | AI-2/LsrR binding rate | 0.5 uM-1 min-1 [2] |
| k4 | Repression coefficient (Lsr-regulator) | 65 uM [2] |
| k5 | REG/AI-2 interaction | 0.0001 uM-1 min-1 [2] |
| kf | AI-2 fluc through alternative pahways | 0.01 uM-1 min-1 [2] |
| kimp | AI-2 import via LasrABCD | 0.01 uM-1 min-1 [2] |
| nOP | Cooperativity coefficient (Lsr-operon) | 4 [2] |
| knR | class="model-th"Cooperativity coefficient (LsrR) | 4 [2] |
| kdeg | Protein decay rate | 0.02 min-1 [2] |
Equations
Equation 1
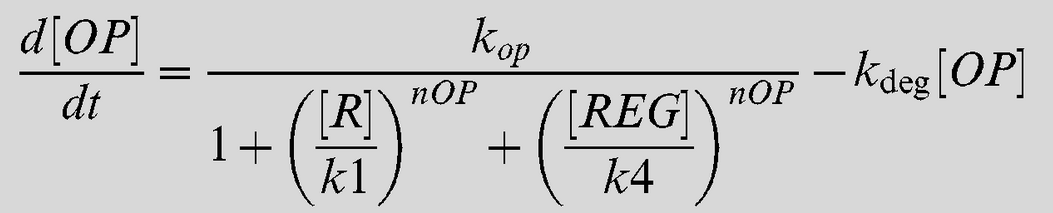
Equation 2
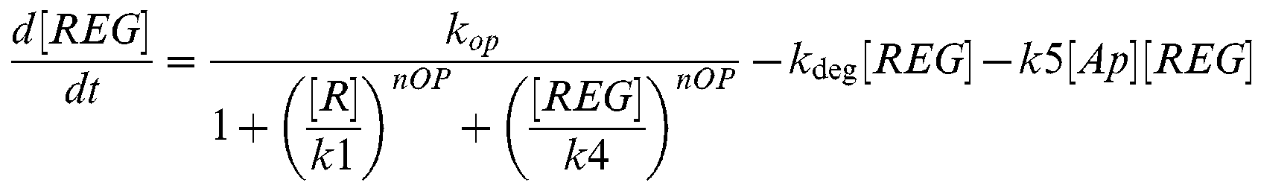
Equation 3
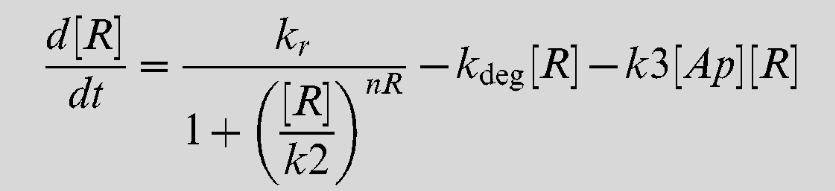
Equation 4
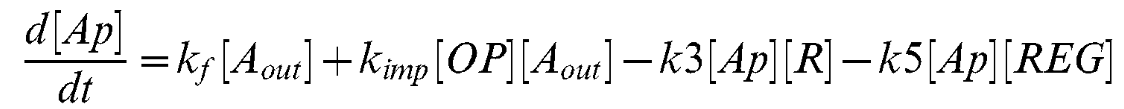
Equation 5

Equation 6
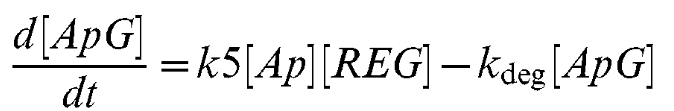
Equation 7
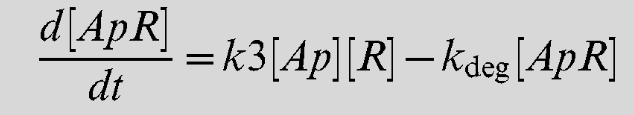
Important notice:
- This part is only the promoter region of LsrA gene which is normally inhibited by LsrR. It is NOT the entire LsrA gene.
- It is INDIRECTLY induced by AI-2, through a complex network involving LsrK,LsrR.
How this promoter works:
By ligating LsrA promoter with a protein coding sequence downstream, we can regulate the expression of that protein by AI-2 as the inducer. To prevent self-inducing by the host cell, the plasmid must be transformed into cell with LuxS gene knocked out.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
//direction/forward
//chassis/prokaryote/ecoli
//promoter
//regulation/positive
| negative_regulators | |
| positive_regulators | 1 |

 1 Registry Star
1 Registry Star
