Part:BBa_K3407022
Short hairpin RNA (shRNA) potential trigger of RNAi. Transcription controlled under T7 promoter.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
shRNAs are single RNA molecules with a strong, self-assembling secondary structure and a loop. In the scientific community, RNA interference (RNAi) terminology can be confusing by referring to different molecules with the same name. The diverse nature of RNA structures makes it difficult to reflect its heterogeneity while keeping a minimum number of terms. Double-stranded RNA (dsRNA) is commonly referred to as complementary sequences of RNA, that hybridise to form a fragment of dsRNA of about 200-600bp [1][2][3][4][5]; others use the term to name shRNAs [6], while some refer to shRNAs as such [7][8]. The reality is, many structures of single-stranded RNAs (ssRNAs) can hybridise with others or themselves to become fully double-stranded, or reserve only a specific part of the whole to form dsRNA.
RNAi is triggered when dsRNA is present in the cytoplasm of most eukaryotes. Dicer is the enzyme in charge of recognising and cleaving dsRNA into either micro RNA (miRNA), or small interfering RNA (siRNA) [9]. In eukaryotes other than plants, siRNA arise from the processing of dsRNA without mismatches, while miRNA are mainly thought to come from mismatched dsRNA processing. In insects, miRNAs are a product of Dicer-1 processing, while siRNA are generated by Dicer-2 [10], at least the latter in a ATP-independent manner [11]. One strand of these small RNAs is selected and loaded in RNA Induced Silencing Complex (RISC), to direct post-transcriptional gene silencing. It is attained by interfering with mRNAs that show a certain degree of complementarity with the selected strand from miRNAs, or by cleaving the mRNA if it shows 100% complementarity with the selected strand from siRNA [12]. This statements remains partially true, as crossovers between the two pathways have been shown to happen, as siRNA can behave like miRNA by interfering with mRNAs with only a certain degree of complementarity, corresponding to a 100% match only with the “seed region” (2-8 nt) of the loaded strand in RISC [13].
RNA hairpins can take both pathways, leading to either siRNA or miRNA. To produce miRNA, a transcript called pri-miRNA is processed by Drosha-DGCR8 complex into pre-miRNA in the nucleus, to later be exported to the cytoplasm by Exportin-5 [14]. To do so, this latter protein needs to recognise the loop of the pre-miRNA and its overhang [15]. Next, pre-miRNA is further processed by Dicer to produce miRNAs [16][17]. On the other hand, when short RNA hairpins do not show any mismatches in their dsRNA sequence, they can be processed by Dicer-2 to produce siRNA [18], as depicted in Figure 1. Interestingly, shRNA can bypass Dicer processing to perform siRNA-like silencing by directly loading into RISC [19]. Therefore, shRNAs are valuable candidates to mediate specific gene silencing through siRNA pathway.
Experimental results
shRNA can be transcribed with T7 RNA polymerase
shRNA are produced in vitro using the T7 RiboMAX RNAi express kit from Promega, which makes use of the viral T7 RNA polymerase.
-
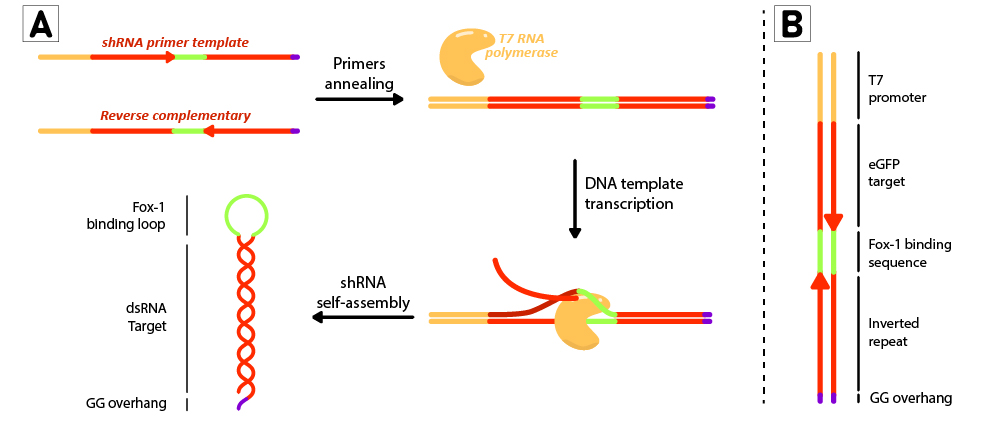 Figure 2: Schematic representation of the shRNA in vitro production using T7 RiboMAX kit from Promega. Primer pairs are annealed in the T4 ligase buffer by heating at 95ºC for 5 minutes and left cooling at room temperature for 30 minutes. Once template is annealed, T7 RNA polymerase is added to transcribe the DNA template at 42ºC for 2 hours. shRNAs self assemble as soon as they are produced. (B) DNA template design for in vitro shRNA production. T7 promoter followed by eGFP target sequences with inverted repeats are linked by the loop region, and finally ends with a GG overhang.
Figure 2: Schematic representation of the shRNA in vitro production using T7 RiboMAX kit from Promega. Primer pairs are annealed in the T4 ligase buffer by heating at 95ºC for 5 minutes and left cooling at room temperature for 30 minutes. Once template is annealed, T7 RNA polymerase is added to transcribe the DNA template at 42ºC for 2 hours. shRNAs self assemble as soon as they are produced. (B) DNA template design for in vitro shRNA production. T7 promoter followed by eGFP target sequences with inverted repeats are linked by the loop region, and finally ends with a GG overhang.
For each short hairpin, a pair of complementary primers were annealed to form a DNA template for transcription (Figure 2B). The DNA template was designed to possess a T7 promoter followed by a 27nt inverted repeat sequence taken from the eGFP gene (nt 78 to 105), and linked together by the 9nt sequence containing the target of Fox-1 RBD (BBa_K3407022). On the 3’ termini, two GG were added to produce the desired overhang, emulating the one left by mini-3 (BBa_K3407002). This leads to a successful transcription without the need to use a terminator (Figure 2A).
-
 Figure 3: Designed short-hairpin RNAs (shRNA). A) Scheme about the different parts of the designed shRNA. B) Urea-PAGE analysis of transcription products from T7 RiboMAX™ Express RNAi System. Primers were annealed as recommended in the kit, and transcription was performed at 42ºC for 2 hours. DNAse treatment was performed as indicated in the kit and the product was purified with MinElute RNA columns (Qiagen). shRNA (BBa_K3407022), shRNA* (BBa_K3407023).
Figure 3: Designed short-hairpin RNAs (shRNA). A) Scheme about the different parts of the designed shRNA. B) Urea-PAGE analysis of transcription products from T7 RiboMAX™ Express RNAi System. Primers were annealed as recommended in the kit, and transcription was performed at 42ºC for 2 hours. DNAse treatment was performed as indicated in the kit and the product was purified with MinElute RNA columns (Qiagen). shRNA (BBa_K3407022), shRNA* (BBa_K3407023).
The commercial kit T7 RiboMAX™ Express RNAi System (Promega) containing the T7 RNA polymerase was used to produce the shRNA hairpins (Figure 3). The expected sizes of the transcript are ~65bp for ssRNA when denatured, and ~32,5bp for dsRNA with its native hairpin structure if the loop is assumed to be part of the dsRNA (which represents an approximation). We would expect the dsRNA ladder to be denatured into ssRNA in the conditions of the gel. On the other hand, shRNA bands are located near 30nt, which indicates the gel denaturing conditions might not have been enough to undo the strong secondary structures of the hairpins, making them unable to move in the gel as their expected ~65 bp ssRNA but rather as ~32,5bp. This phenomenon is commonly seen when studying hairpins in Urea-PAGE assays [20]. We conclude both shRNA variants have successfully been transcribed in vitro and they can be used for binding assays with the Fox-1 RBD protein and Dicer processing experiments.
Fox-1 RBD binds to shRNA
To study RNA-protein interaction, an Electrophoretic mobility shift assay (EMSA) was performed . When a protein binds to RNA, the complex migrates slower in the gel, shifting the RNA band to a higher position. shRNA (BBa_K3407022) and Fox-1 RBD (BBa_K3407020) were first incubated in 1:1 and 1:2 molar ratios shRNA:Fox in binding buffer. 400ng of shRNA were loaded in each lane corresponding to 10 fmol, along with 10 fmol of Fox-1 RBD (1:1 ratio) or 20 fmol (1:2 ratio) in a total of 15 μL reaction. To demonstrate binding specificity, the same experiment was performed with Fox-1 RBD* (BBa_K3407024), a mutated version of Fox-1 with a reduced affinity for its binding sequence. In addition, the assays were also carried out with shRNA* (BBa_K3407023), a version of the shRNA with two mutations in the loop, which inhibits binding to Fox-1 RBD [21] (Figure 4). Samples were then loaded in a native polyacrylamide gel to avoid protein denaturation (Figure 5).
-
 Figure 4: Illustration of Fox-1 domains used for the assay and their expected interaction with the target sequence of the shRNA or shRNA*. Mutations in either proteins or RNA are depicted as red dots. Mutated sequences of RNA or proteins are colored in orange, while wt protein domains Fox-1 RBD and wt binding sequence in the loop are colored in green.
Figure 4: Illustration of Fox-1 domains used for the assay and their expected interaction with the target sequence of the shRNA or shRNA*. Mutations in either proteins or RNA are depicted as red dots. Mutated sequences of RNA or proteins are colored in orange, while wt protein domains Fox-1 RBD and wt binding sequence in the loop are colored in green.
-
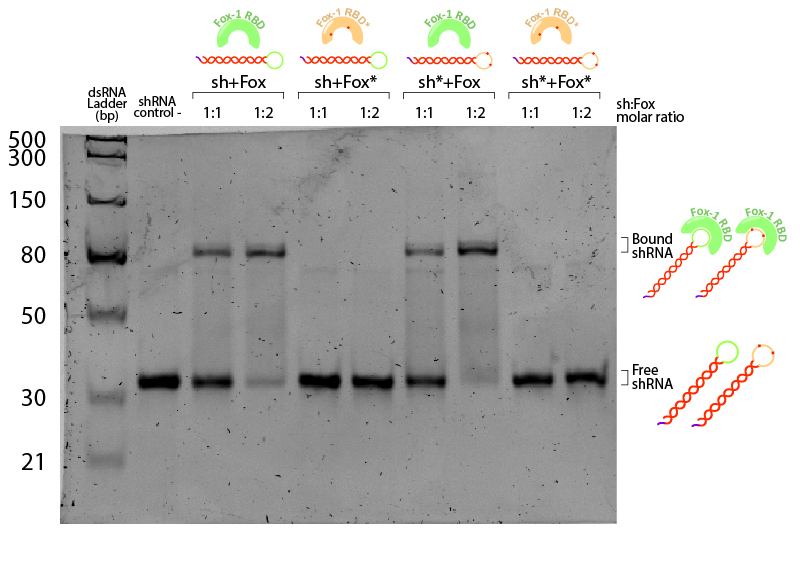 Figure 5:EMSA assay of Fox-1 RBD binding to shRNA. Stars (*) indicate mutated versions of the molecules, where sh* is an shRNA with two mutations in the loop region recognised by Fox-1 RBD (G6A and C3U), and Fox* is the RBD of Fox-1 with two mutations (F160A and F126A) rendering it unable to recognise the RNA target sequence. Control (-) is shRNA incubated in the same conditions without Fox. Incubation was performed in the binding buffer (10 mM Tris pH 7,5; 1 mM EDTA; 100 mM KCl; 0.1 mM DTT; 5% Glycerol; 0.01 mg/ml BSA) in different molar ratios at room temperature for one hour. Electrophoresis with 19:1 (Acrylamide:Bisacrylamide) 12% gel in TAE buffer. Electrophoresis was run at 120 V for about 3 hours in a TAE 1x buffer, stained with Sybr Safe staining solution (0.1% vol./vol. Sybr Safe in TAE buffer).
Figure 5:EMSA assay of Fox-1 RBD binding to shRNA. Stars (*) indicate mutated versions of the molecules, where sh* is an shRNA with two mutations in the loop region recognised by Fox-1 RBD (G6A and C3U), and Fox* is the RBD of Fox-1 with two mutations (F160A and F126A) rendering it unable to recognise the RNA target sequence. Control (-) is shRNA incubated in the same conditions without Fox. Incubation was performed in the binding buffer (10 mM Tris pH 7,5; 1 mM EDTA; 100 mM KCl; 0.1 mM DTT; 5% Glycerol; 0.01 mg/ml BSA) in different molar ratios at room temperature for one hour. Electrophoresis with 19:1 (Acrylamide:Bisacrylamide) 12% gel in TAE buffer. Electrophoresis was run at 120 V for about 3 hours in a TAE 1x buffer, stained with Sybr Safe staining solution (0.1% vol./vol. Sybr Safe in TAE buffer).
The non-bound shRNA and shRNA* appear on the gel as ~30bp dsRNA (negative control, Figure 5). Incubation of shRNA and ahRNA* with wild-type Fox-1 RBD leads to a notable shift of the RNA band at both 1:1 and 1:2 molar ratios. This upper band corresponds to the shRNA-Fox-1 RBD complex, showing that both purified compounds are active. The lower fraction of free shRNA in the 1:2 molar ratio indicates that increasing the concentration of Fox-1 RBD yields a higher amount of complex formed.
Interestingly, incubation of shRNA with mutated Fox* RBD does not lead to complex formation. This results corroborates literature data showing that the introduced mutations strongly reduce the affinity of the protein to the target RNA sequence. Surprisingly, Fox-1 RBD (denoted as Fox), but not Fox-1 RBD* (denoted as Fox*), is also able to bind to mutated shRNA*, suggesting that under the studied conditions the mutations in the RNA loop sequence do not abolish complex formation (Figure 6). Different results were previously obtained using surface plasmon resonance [22], which may be explained by differences in the experimental conditions.
-
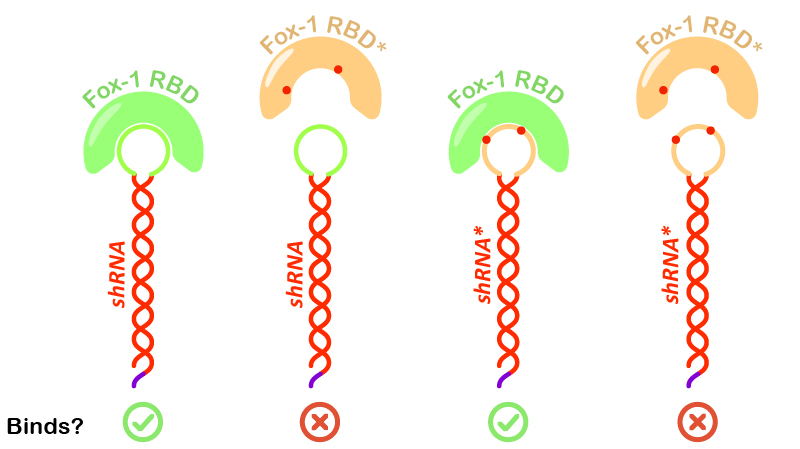 Figure 6:Illustration of Fox-1 domains used for the assay and their obtained results from interaction assay with the target sequence of the shRNA or shRNA*. Mutations in either proteins or RNA are depicted as red dots. Mutated sequences of RNA or proteins are colored in orange, while wt protein domains Fox-1 RBD and wt binding sequence in the loop are colored in green.
Figure 6:Illustration of Fox-1 domains used for the assay and their obtained results from interaction assay with the target sequence of the shRNA or shRNA*. Mutations in either proteins or RNA are depicted as red dots. Mutated sequences of RNA or proteins are colored in orange, while wt protein domains Fox-1 RBD and wt binding sequence in the loop are colored in green.
Hairpins with GG 3’ overhangs are suitable Dicer substrates
Dicer processing is the first step of the RNAi-mediated gene silencing where small interfering RNAs (siRNAs) are produced. Dicer-2 isoform products are later used to cleave messenger RNAs (mRNAs) with a 100% complementarity with one of the siRNA’s strand [23]. Not all dsRNA structures are processed with the same efficiency due to the preferences Dicer shows towards certain substrates. For instance, dsRNA sequences embedded in a longer RNA transcript or with capped ends have been reported to show less processability than those at the termini, which generally show an increased siRNA production rate when ending with 3’ overhangs in absence of ATP [24]. In particular, GG overhangs have shown a medium level of processivity among all possible combinations in humans.
With this in mind, we decided to study whether GG protruding 3’ overhangs emulating those left by mini-3 cleavage are suitable for the production of free, mature shRNA with RNAi triggering potential. shRNAs were designed with the aforementioned overhang and their processivity was tested with a Dicer cleaving assay using purified Dicer-2 from the insect species Drosophila melanogaster (Figure 7), kindly provided by TU Delft BN department.
-
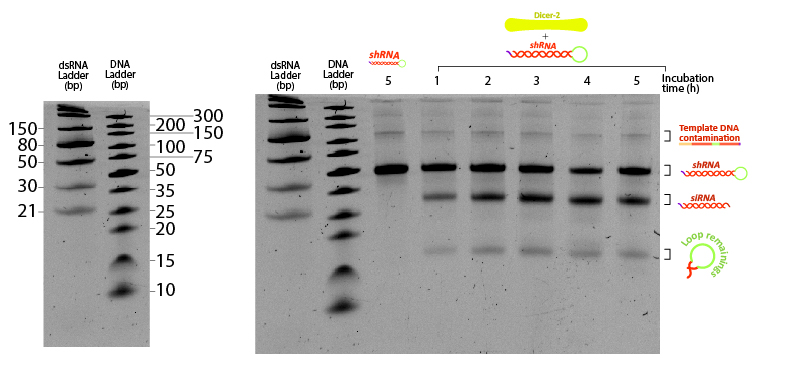 Figure 7:Figure 7: shRNA processed by Dicer at different incubation times. Two ladders were added to compare the mobility of dsRNA with conventional DNA ladder. 400 ng of shRNA were incubated at 25ºC in Dicer buffer (Tris pH 7.5 10 mM, MgCl2 1 mM, NaCl 37.5 mM, Glycerol 5%) with 0.2 µM Dicer. Each tube was snap-frozen at the desired time point with liquid nitrogen until the end of the assay (5 hours). Control (-) sample consists of shRNA incubated 5 hours under the same buffer and temperature conditions without Dicer. Urea-PAGE 15% of 19:1 (Acrylamide:Bisacrylamide) ratio. Electrophoresis ran for ~3 hours at 120 V.
Figure 7:Figure 7: shRNA processed by Dicer at different incubation times. Two ladders were added to compare the mobility of dsRNA with conventional DNA ladder. 400 ng of shRNA were incubated at 25ºC in Dicer buffer (Tris pH 7.5 10 mM, MgCl2 1 mM, NaCl 37.5 mM, Glycerol 5%) with 0.2 µM Dicer. Each tube was snap-frozen at the desired time point with liquid nitrogen until the end of the assay (5 hours). Control (-) sample consists of shRNA incubated 5 hours under the same buffer and temperature conditions without Dicer. Urea-PAGE 15% of 19:1 (Acrylamide:Bisacrylamide) ratio. Electrophoresis ran for ~3 hours at 120 V.
The unprocessed shRNA gives a clear band on the gel, slightly above the 30nt size marker of the dsRNA ladder (Figure 7). Incubation with Dicer yields two lower bands, likely corresponding to the two cleavage products: the siRNA and the loop. Increasing the reaction duration from 1 hour to longer times is accompanied by a higher fraction of both enzymatic products. These results demonstrate that the in vitro transcribed shRNA containing GG 3’ overhangs can be processed by Dicer from the insect Drosophila melanogaster, adding evidence that our shRNAs have potential to silence any targeted gene from the locusts by means of RNAi.
References
- Lenaerts, C., Palmans, J., Marchal, E., Verdonck, R. and Vanden Broeck, J., 2020. Role Of The Venus Kinase Receptor In The Female Reproductive Physiology Of The Desert Locust, Schistocerca Gregaria. Sci Rep 7.
- Lenaerts, C., Marchal, E., Peeters, P. and Vanden Broeck, J., 2020. The Ecdysone Receptor Complex Is Essential For The Reproductive Success In The Female Desert Locust, Schistocerca Gregaria. 9
- Marchal, E., Badisco, L., Verlinden, H., Vandersmissen, T., Van Soest, S., Van Wielendaele, P. and Vanden Broeck, J., 2020. Role Of The Halloween Genes, Spook And Phantom In Ecdysteroidogenesis In The Desert Locust, Schistocerca Gregaria.
- Vatanparast, M. and Kim, Y., 2020. Optimization Of Recombinant Bacteria Expressing Dsrna To Enhance Insecticidal Activity Against A Lepidopteran Insect, Spodoptera Exigua.
- Adeyinka, O., Tabassum, B., Nasir, I., Yousaf, I., Sajid, I., Shehzad, K., Batcho, A. and Husnain, T., 2020. Identification And Validation Of Potential Reference Gene For Effective Dsrna Knockdown Analysis In Chilo Partellus.
- Ma, Z., Zhou, H., Wei, Y., Yan, S. and Shen, J., 2020. A Novel Plasmid– Escherichia Coli System Produces Large Batch Dsrnas For Insect Gene Silencing.
- Yuka M., Rina K., Hiroyuki S., Tamaki E., and Takashi O., 2011. Photosensitizing Carrier Proteins for Photoinducible RNA Interference.
- Soler Canton A., Danelon C., Dogterom A., 2020. Synthetic Biology Meets Liposome-Based Drug Delivery. [online] Repository.tudelft.nl.
- Zhang, H., Kolb, F., Jaskiewicz, L., Westhof, E. and Filipowicz, W., 2020. Single Processing Center Models For Human Dicer And Bacterial Rnase III.
- Young S., Kenji N., John W., Kevin K., Zhengying H., Erik J., Richard W., 2004. Distinct Roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA Silencing Pathways.
- Sinha, N., Trettin, K., Aruscavage, P. and Bass, B., 2020. Drosophila Dicer-2 Cleavage Is Mediated By Helicase- And Dsrna Termini-Dependent States That Are Modulated By Loquacious-PD.
- Lam, J., Chow, M., Zhang, Y. and Leung, S., 2020. Sirna Versus Mirna As Therapeutics For Gene Silencing. 9
- Burchard, J., Jackson, A., Malkov, V., Needham, R., Tan, Y., Bartz, S., Dai, H., Sachs, A. and Linsley, P., 2020. Microrna-Like Off-Target Transcript Regulation By Sirnas Is Species Specific.
- Bofill-De Ros, X. and Gu, S., 2020. Guidelines For The Optimal Design Of Mirna-Based Shrnas.
- Wang, X., Xu, X., Ma, Z., Huo, Y., Xiao, Z., Li, Y. and Wang, Y., 2020. Dynamic Mechanisms For Pre-Mirna Binding And Export By Exportin-5.
- Bofill-De Ros, X. and Gu, S., 2020. Guidelines For The Optimal Design Of Mirna-Based Shrnas.
- Lam, J., Chow, M., Zhang, Y. and Leung, S., 2020. Sirna Versus Mirna As Therapeutics For Gene Silencing.
- Sioud, M., 2020. What Are The Key Targeted Delivery Technologies Of Sirna Now?.
- Liu, Y., Schopman, N. and Berkhout, B., 2020. Dicer-Independent Processing Of Short Hairpin Rnas.
- Soler Canton, A. (2019) Synthetic biology meets liposome-based drug delivery. Casimir PhD series, Delft-Leiden 2019-35.
- Liu, Y., Schopman, N. and Berkhout, B., 2020. Dicer-Independent Processing Of Short Hairpin Rnas.
- Lam, J. K. W., Chow, M. Y. T., Zhang, Y., & Leung, S. W. S. (2015). siRNA versus miRNA as therapeutics for gene silencing. Molecular Therapy - Nucleic Acids.
- Sinha, N. K., Trettin, K. D., Aruscavage, P. J., & Bass, B. L. (2015). Drosophila dicer-2 cleavage is mediated by helicase- and dsrna termini-dependent states that are modulated by loquacious-PD. Molecular Cell.
- Vermeulen, A., Behlen, L., Reynolds, A., Wolfson, A., Marshall, W. S., Karpilow, J., & Khvorova, A. (2005). The contributions of dsRNA structure to Dicer specificity and efficiency. RNA.
| None |

