Part:BBa_K2715008
Antisense RNA targeting C. difficile toxins composite 2
Usage and Biology
This part is designed to suppress the production of toxins TcdA and TcdB in Clostridium difficile. Toxin production is inhibited via an antisense RNA strategy whereby the translation of toxin mRNA is reduced.
Synthetic antisense RNA parts BBa_K2715018 and BBa_K2715016 are transcribed from strong promoters BBa_K2715010 and BBa_K2715011 respectively. Both antisense RNA parts are synthetic and were designed to target two important C. difficile toxins TcdA (part BBa_K2715016) and TcdB (part BBa_K2715018). The first antisense RNA BBa_K2715018 targets TcdB and is transcribed via promoter BBa_K2715010 which was taken from Clostridium acetobutylicum where it natively controls expression of a thiolase gene. The second antisense RNA BBa_K2715015 targets TcdA and is transcribed via promoter BBa_K2715011 which was taken from Clostridium sporogenes where it natively controls expression of a ferredoxin gene. Each of these promoters were designed to omit the existing 5’UTR of the native gene and as such the transcriptional start site is predicted to be at the start of the relevant asRNA part. In between the two promoter-asRNA pairs is a transcriptional terminator BBa_K2715014 which is native to C. acetobutylicum where it is situated between the pyrE and hydA genes.
Characterisation
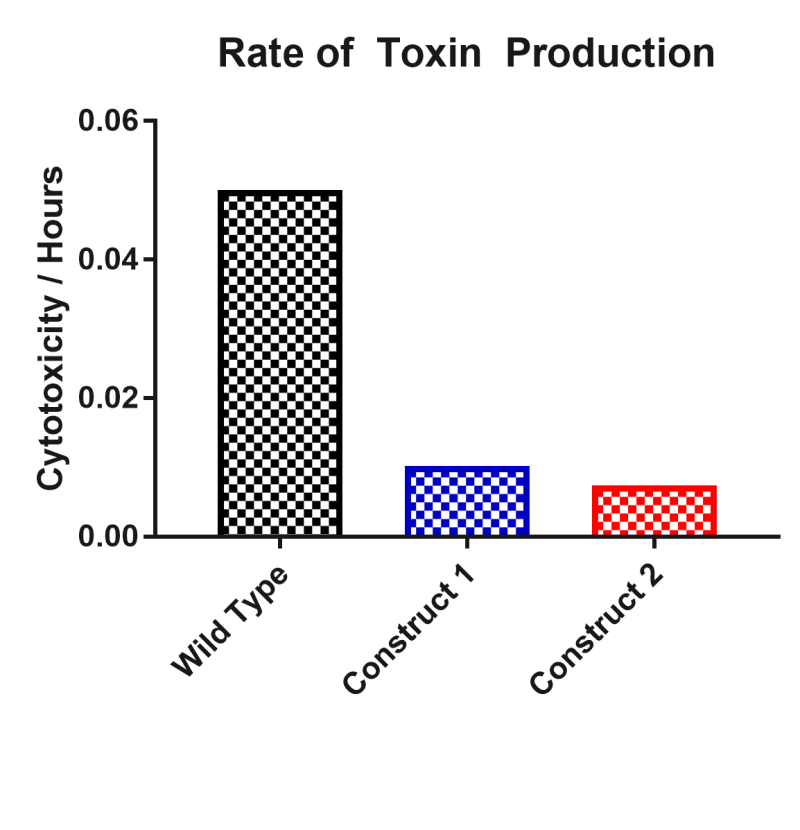
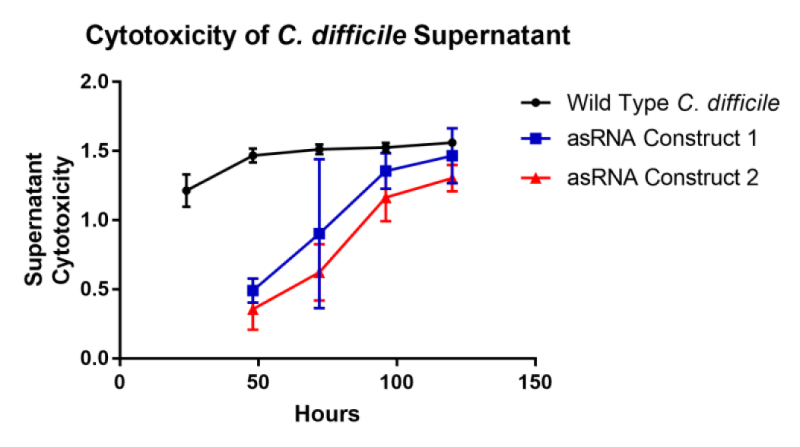
This part was characterised in terms of the extent to which it inhibited toxin production in C. difficile. The part was introduced into C. difficile on a replicative plasmid and supernatant samples were assayed for their level of cytotoxicity on African green monkey kidney epithelial ‘Vero’ cells. Compared to wild type C. difficile, this part reduced the rate of toxin production by 85%. Samples were taken daily for five days and applied to Vero cells with a lactate dehydrogenase assay providing an estimate of cytotoxicity. From these results an estimate of toxin production was calculated from cytotoxicity divided by time.
Conclusions
Two asRNA constructs were cloned named ‘asRNA Construct One’ [Bba_K2715007] and ‘asRNA Construct Two’ [Bba_K2715008]. Both of these constructs target both toxin genes TcdA and TcdB with asRNA parts of varying length with asRNA Construct Two having longer regions of homology with 50bp of coding region verses 24bp for asRNA Construct One. These constructs were designed with the promoter results in mind, selecting the suspected two strongest promoters in C. difficile. Both constructs were assessed in terms of their ability to reduce C. difficile culture supernatant cytotoxicity on mammalian ‘Vero’ cells. The rate of toxin production was decreased by 80% and 85% by asRNA Construct One and asRNA Construct Two respectively. The main conclusion to draw from this result is that an asRNA strategy is viable for reducing C. difficile strain toxicity. Another conclusion of note is that having a longer region of homology with the target gene does seem to impact on the effectiveness of suppression significantly since asRNA Construct Two has a 5% greater effect whilst having 26bp extra of homology per toxin gene.
References
1. Heap, J. T., Pennington, O. J., Cartman, S. T. & Minton, N. P. A modular system for Clostridium shuttle plasmids. J. Microbiol. Methods 78, 79–85 (2009).
2. Ammerman, Nicole, C. ., Beier-Sexton, M. & Azad, Abdu, F. . Growth and Maintenance of Vero Cell Lines. Curr. Protoc. Microbiol. 1–10 (2009). doi:10.1002/9780471729259.mca04es11.Growth
3. Liu, Y. W., Chen, Y. H., Chen, J. W., Tsai, P. J. & Huang, I. H. Immunization with recombinant TcdB-encapsulated nanocomplex induces protection against Clostridium difficile challenge in a mouse model. Front. Microbiol. 8, 1–14 (2017).
4. Kuehne, S. A. et al. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467, 711–713 (2010).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |